The long-term safety of topical corticosteroids in atopic dermatitis: A systematic review
- PMID: 37799373
- PMCID: PMC10549798
- DOI: 10.1002/ski2.268
The long-term safety of topical corticosteroids in atopic dermatitis: A systematic review
Abstract
Background: Topical corticosteroids (TCS) are a first-line treatment for eczema, but there are concerns about their safety when used long-term.
Objectives: To systematically review adverse effects associated with longer-term use of TCS for eczema.
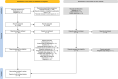
Methods: Randomised controlled trials (RCTs), cohort and case-control studies reporting adverse effects of TCS (comparators: no TCS treatment, other topicals) in patients with eczema were identified. Included studies had greater than one year of follow-up, minimum cohort size of 50 participants, or minimum 50 per arm for RCTs. Evidence was GRADE-assessed. Prospero registration CRD42021286413.
Results: We found seven studies (two randomised, five observational); two RCTs (n = 2570, including 1288 receiving TCS), two cohort (all received TCS n = 148) and three case-control studies (cases n = 10 322, controls n = 12 201). Evidence from two RCTS (n = 2570, children, three and five years' duration) comparing TCS to topical calcineurin inhibitors found intermittent TCS use probably results in little to no difference in risk of growth abnormalities, non-skin infections, impaired vaccine response and lymphoma/non lymphoma malignancies. The five-year RCT reported only one episode of skin atrophy (n = 1213 TCS arm; mild/moderate potency), suggesting TCS use probably results in little to no difference in skin thinning when used intermittently to treat flares. No cases of clinical adrenal insufficiency were reported in 75 patients using mild/moderate TCS in the three-year RCT. Small associations between TCS and type-2 diabetes and lymphoma were identified in two case-control studies compared to no TCS, but the evidence is very uncertain. No long-term studies concerning topical steroid withdrawal or eye problems were identified.
Conclusion: This review provides some reassuring data on growth and skin thinning when TCS are used intermittently for up to 5 years, but many knowledge gaps remain.
© 2023 The Authors. Skin Health and Disease published by John Wiley & Sons Ltd on behalf of British Association of Dermatologists.
Conflict of interest statement
Jane Harvey, Stephanie J. Lax, have declared that they have no conflict of interest. Alison Lowe has worked for AbbVie and has been sponsored to attend conference and educational events by AbbVie and Eli‐Lilly. Sandra Lawton is funded by an honorarium (Thorton and Ross – lecture). Sandra Lawton took part in a Podcast transitioning young people with Eczema (funded by Abbvie). Sinead M. Langan is funded by a Wellcome Senior Clinical fellowship. Sinead M. Langan is an investigator on the European Union Horizon 2020‐funded BIOMAP Consortium (http://www.biomap‐imi.eu/), but not in receipt of industry funding. Miriam Santer, Sandra Lawton, Sinead M. Langan, Amanda Roberts, Beth Stuart, Hywel C. Williams and Kim S. Thomas are ECO co‐applicants. HCW and KST are members of the Harmonising Outcomes Measures for Eczema Executive Group.
Figures
References
-
- Johansson SG, Bieber T, Dahl R, Friedmann PS, Lanier BQ, Lockey RF, et al. Revised nomenclature for allergy for global use: report of the nomenclature review committee of the world allergy organization, october 2003. J Allergy Clin Immunol. 2004;113(5):832–836. 10.1016/j.jaci.2003.12.591 - DOI - PubMed
-
- Wollenberg A, Kinberger M, Arents B, Aszodi N, Avila Valle G, Barbarot S, et al. European guideline (EuroGuiDerm) on atopic eczema – part II: non‐systemic treatments and treatment recommendations for special AE patient populations. J Eur Acad Dermatol Venereol. 2022;36(11):1904–1926. 10.1111/jdv.18429 - DOI - PubMed
-
- Eckert L, Gupta S, Amand C, Gadkari A, Mahajan P, Gelfand JM. Impact of atopic dermatitis on health‐related quality of life and productivity in adults in the United States: an analysis using the National Health and Wellness Survey. J Am Acad Dermatol. 2017;77(2):274–279.e3. 10.1016/j.jaad.2017.04.019 - DOI - PubMed
LinkOut - more resources
Full Text Sources