Advanced Combination Treatment With Biologic Agents and Novel Small Molecule Drugs for Inflammatory Bowel Disease
- PMID: 37799456
- PMCID: PMC10548249
Advanced Combination Treatment With Biologic Agents and Novel Small Molecule Drugs for Inflammatory Bowel Disease
Abstract
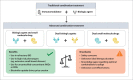
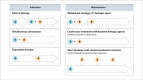
The use of combination therapy with a biologic agent and immunosuppressant has well-established efficacy and safety and is common practice in the management of inflammatory bowel disease (IBD). Current research has shifted focus toward the use of advanced combination treatment (ACT). This term was coined to describe combination therapy using 2 or more advanced treatments (biologic agents and/or oral small molecule drugs) with the aim of achieving optimal disease control in selected patients. An ACT approach may be particularly beneficial in patients with documented medically refractory IBD and in patients with a poor prognosis, extraintestinal manifestations, or concomitant immune-mediated inflammatory diseases. To date, the body of evidence for ACT strategies in IBD is largely comprised of uncontrolled retrospective case series and cohort studies in highly refractory patients. Recently, results from the VEGA trial have suggested that combination induction therapy with guselkumab and golimumab was more effective in ulcerative colitis than either agent alone. However, questions remain about issues such as related costs, ACT duration, and optimal combinations to adopt. Future randomized controlled trials are likely to evaluate rationally selected combinations of agents. This article summarizes the available literature on ACT, including comparisons with traditional combination therapy and the rheumatology field, and discusses practical recommendations, profiles of IBD patients who should be considered for combination approaches in clinical practice, and remaining knowledge gaps.
Keywords: Advanced combination treatment; Crohn’s disease; dual biologic therapy; dual targeted therapy; inflammatory bowel disease; ulcerative colitis.
Copyright © 2023, Gastro-Hep Communications, Inc.
Figures
References
-
- Hanauer SB, Feagan BG, Lichtenstein GR et al. ACCENT I Study Group. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359(9317):1541–1549. - PubMed
-
- Colombel JF, Sandborn WJ, Rutgeerts P et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology. 2007;132(1):52–65. - PubMed
-
- Feagan BG, Sandborn WJ, Gasink C et al. UNITI–IM-UNITI Study Group. Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2016;375(20):1946–1960. - PubMed
-
- Schett G, McInnes IB, Neurath MF. Reframing immune-mediated inflammatory diseases through signature cytokine hubs. N Engl J Med. 2021;385(7):628–639. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
