A periosteum-derived cell line to study the role of BMP/TGFβ signaling in periosteal cell behavior and function
- PMID: 37799511
- PMCID: PMC10547901
- DOI: 10.3389/fphys.2023.1221152
A periosteum-derived cell line to study the role of BMP/TGFβ signaling in periosteal cell behavior and function
Abstract
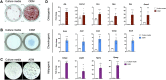
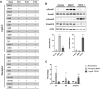
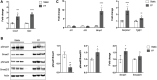
The periosteum is a thin tissue surrounding each skeletal element that contains stem and progenitor cells involved in bone development, postnatal appositional bone growth, load-induced bone formation, and fracture repair. BMP and TGFβ signaling are important for periosteal activity and periosteal cell behavior, but thorough examination of the influence of these pathways on specific cell populations resident in the periosteum is lacking due to limitations associated with primary periosteal cell isolations and in vitro experiments. Here we describe the generation of a novel periosteum-derived clonal cell (PDC) line from postnatal day 14 mice and use it to examine periosteal cell behavior in vitro. PDCs exhibit key characteristics of periosteal cells observed during skeletal development, maintenance, and bone repair. Specifically, PDCs express established periosteal markers, can be expanded in culture, demonstrate the ability to differentiate into chondrocytes, osteoblasts, and adipocytes, and exhibit an osteogenic response to physical stimulation. PDCs also engage in BMP and/or TGFβ signaling when treated with the activating ligands BMP2 and TGFβ-1, and in response to mechanical stimulation via fluid shear. We believe that this PDC line will be useful for large-scale, long-term experiments that were not feasible when using primary periosteal cells. Anticipated future uses include advancing our understanding of the signaling interactions that occur during appositional bone growth and fracture repair and developing drug screening platforms to discover novel growth and fracture healing factors.
Keywords: BMP signaling; TGFβ signaling; cell line; mechanotransduction; periosteum.
Copyright © 2023 Moore, Maridas, Gamer, Chen, Burton and Rosen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Periosteal progenitors contribute to load-induced bone formation in adult mice and require primary cilia to sense mechanical stimulation.Stem Cell Res Ther. 2018 Jul 11;9(1):190. doi: 10.1186/s13287-018-0930-1. Stem Cell Res Ther. 2018. PMID: 29996901 Free PMC article.
-
Low-Intensity Pulsed Ultrasound Stimulates Osteogenic Differentiation of Periosteal Cells In Vitro.Tissue Eng Part A. 2021 Jan;27(1-2):63-73. doi: 10.1089/ten.TEA.2019.0331. Epub 2020 May 21. Tissue Eng Part A. 2021. PMID: 32164486
-
Osteogenic Differentiation of Periosteal Cells During Fracture Healing.J Cell Physiol. 2017 May;232(5):913-921. doi: 10.1002/jcp.25641. Epub 2016 Oct 26. J Cell Physiol. 2017. PMID: 27731505 Free PMC article. Review.
-
Sox9 positive periosteal cells in fracture repair of the adult mammalian long bone.Bone. 2017 Oct;103:12-19. doi: 10.1016/j.bone.2017.06.008. Epub 2017 Jun 13. Bone. 2017. PMID: 28627474 Free PMC article.
-
Periosteal Skeletal Stem and Progenitor Cells in Bone Regeneration.Curr Osteoporos Rep. 2022 Oct;20(5):334-343. doi: 10.1007/s11914-022-00737-8. Epub 2022 Jul 13. Curr Osteoporos Rep. 2022. PMID: 35829950 Review.
Cited by
-
Periosteum Containing Implicit Stem Cells: A Progressive Source of Inspiration for Bone Tissue Regeneration.Int J Mol Sci. 2024 Feb 10;25(4):2162. doi: 10.3390/ijms25042162. Int J Mol Sci. 2024. PMID: 38396834 Free PMC article. Review.
-
Promoting bone callus formation by taking advantage of the time-dependent fracture gap strain modulation.Front Surg. 2024 May 2;11:1376441. doi: 10.3389/fsurg.2024.1376441. eCollection 2024. Front Surg. 2024. PMID: 38756355 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

