Chirality: a key parameter in chemical probes
- PMID: 37799583
- PMCID: PMC10549247
- DOI: 10.1039/d3cb00082f
Chirality: a key parameter in chemical probes
Abstract
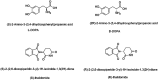
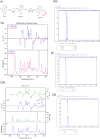
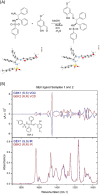
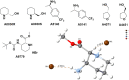
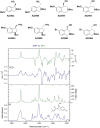
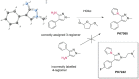
Many small molecule bioactive and marketed drugs are chiral. They are often synthesised from commercially available chiral building blocks. However, chirality is sometimes incorrectly assigned by manufacturers with consequences for the end user ranging from: experimental irreproducibility, wasted time on synthesising the wrong product and reanalysis, to the added cost of purchasing the precursor and resynthesis of the correct stereoisomer. Further on, this could lead to loss of reputation, loss of funding, to safety and ethical concerns due to potential in vivo administration of the wrong form of a drug. It is our firm belief that more stringent control of chirality be provided by the supplier and, if needed, requested by the end user, to minimise the potential issues mentioned above. Certification of chirality would bring much needed confidence in chemical structure assignment and could be provided by a variety of techniques, from polarimetry, chiral HPLC, using known chiral standards, vibrational circular dichroism, and x-ray crystallography. A few case studies of our brushes with wrong chirality assignment are shown as well as some examples of what we believe to be good practice.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
Similar articles
-
X-ray and Optical Circular Dichroism as Local and Global Ultrafast Chiral Probes of [12]Helicene Racemization.J Am Chem Soc. 2023 Sep 27;145(38):21012-21019. doi: 10.1021/jacs.3c07032. Epub 2023 Sep 13. J Am Chem Soc. 2023. PMID: 37704187
-
Assignment of absolute stereostructures through quantum mechanics electronic and vibrational circular dichroism calculations.J Asian Nat Prod Res. 2016;18(1):72-91. doi: 10.1080/10286020.2015.1134502. J Asian Nat Prod Res. 2016. PMID: 26880597
-
Chiral Spectroscopy of Nanostructures.Acc Chem Res. 2023 Jun 20;56(12):1359-1372. doi: 10.1021/acs.accounts.2c00756. Epub 2023 May 31. Acc Chem Res. 2023. PMID: 37256726
-
Vibrational Circular Dichroism Spectroscopy of Chiral Molecular Crystals: Insights from Theory.Angew Chem Int Ed Engl. 2023 Oct 9;62(41):e202303595. doi: 10.1002/anie.202303595. Epub 2023 Jul 3. Angew Chem Int Ed Engl. 2023. PMID: 37071543 Review.
-
Chiral Gold Nanoclusters: Atomic Level Origins of Chirality.Chem Asian J. 2017 Aug 4;12(15):1839-1850. doi: 10.1002/asia.201700023. Epub 2017 Jun 27. Chem Asian J. 2017. PMID: 28653468 Review.
Cited by
-
Stereo-Differentiating Asymmetric Rh(I)-Catalyzed Pauson-Khand Reaction: A DFT-Informed Approach to Thapsigargin Stereoisomers.J Am Chem Soc. 2025 Jan 8;147(1):498-509. doi: 10.1021/jacs.4c11661. Epub 2024 Dec 19. J Am Chem Soc. 2025. PMID: 39702925 Free PMC article.
References
-
- Arrowsmith C. H. Audia J. E. Austin C. Baell J. Bennett J. Blagg J. Bountra C. Brennan P. E. Brown P. J. Bunnage M. E. Buser-Doepner C. Campbell R. M. Carter A. J. Cohen P. Copeland R. A. Cravatt B. Dahlin J. L. Dhanak D. Edwards A. M. Frederiksen M. Frye S. V. Gray N. Grimshaw C. E. Hepworth D. Howe T. Huber K. V. Jin J. Knapp S. Kotz J. D. Kruger R. G. Lowe D. Mader M. M. Marsden B. Mueller-Fahrnow A. Müller S. O'Hagan R. C. Overington J. P. Owen D. R. Rosenberg S. H. Roth B. Ross R. Schapira M. Schreiber S. L. Shoichet B. Sundström M. Superti-Furga G. Taunton J. Toledo-Sherman L. Walpole C. Walters M. A. Willson T. M. Workman P. Young R. N. Zuercher W. J. Nat. Chem. Biol. 2015;11:536–541. doi: 10.1038/nchembio.1867. - DOI - PMC - PubMed
-
- Schnider P., The Medicinal Chemist's Guide to Solving ADMET Challenges, Royal Society of Chemistry, 2021
Publication types
LinkOut - more resources
Full Text Sources

