Microrheology and structural quantification of hypercoagulable clots
- PMID: 37799698
- PMCID: PMC10549726
- DOI: 10.1364/BOE.492669
Microrheology and structural quantification of hypercoagulable clots
Abstract
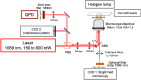
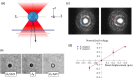
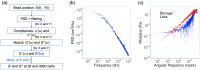
Hypercoagulability is a pathology that remains difficult to explain today in most cases. It is likely due to a modification of the conditions of polymerization of the fibrin, the main clot component. Using passive microrheology, we measured the mechanical properties of clots and correlated them under the same conditions with structural information obtained with confocal microscopy. We tested our approach with known alterations: an excess of fibrinogen and of coagulation Factor VIII. We observed simultaneously a rigidification and densification of the fibrin network, showing the potential of microrheology for hypercoagulability diagnosis.
© 2023 Optica Publishing Group under the terms of the Optica Open Access Publishing Agreement.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Leong L., Chernysh I. N., Xu Y., Sim D., Nagaswami C., de Lange Z., Kosolapova S., Cuker A., Kauser K., Weisel J. W., “Clot stability as a determinant of effective factor VIII replacement in hemophilia A,” Research and Practice in Thrombosis and Haemostasis 1(2), 231–241 (2017).10.1002/rth2.12034 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
