Isotope effects in dynamics of water isotopologues induced by core ionization at an x-ray free-electron laser
- PMID: 37799711
- PMCID: PMC10550338
- DOI: 10.1063/4.0000197
Isotope effects in dynamics of water isotopologues induced by core ionization at an x-ray free-electron laser
Abstract
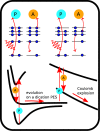
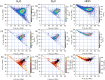
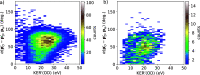
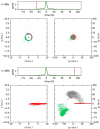
Dynamical response of water exposed to x-rays is of utmost importance in a wealth of science areas. We exposed isolated water isotopologues to short x-ray pulses from a free-electron laser and detected momenta of all produced ions in coincidence. By combining experimental results and theoretical modeling, we identify significant structural dynamics with characteristic isotope effects in H2O2+, D2O2+, and HDO2+, such as asymmetric bond elongation and bond-angle opening, leading to two-body or three-body fragmentation on a timescale of a few femtoseconds. A method to disentangle the sequences of events taking place upon the consecutive absorption of two x-ray photons is described. The obtained deep look into structural properties and dynamics of dissociating water isotopologues provides essential insights into the underlying mechanisms.
© 2023 Author(s).
Conflict of interest statement
The authors have no conflicts to disclose.
Figures







Similar articles
-
On the nature and origin of dicationic, charge-separated species formed in liquid water on X-ray irradiation.Nat Chem. 2013 Jul;5(7):590-6. doi: 10.1038/nchem.1680. Epub 2013 Jun 20. Nat Chem. 2013. PMID: 23787749
-
Ultrafast X-ray Transient Absorption Spectroscopy of Gas-Phase Photochemical Reactions: A New Universal Probe of Photoinduced Molecular Dynamics.Acc Chem Res. 2018 Dec 18;51(12):3203-3211. doi: 10.1021/acs.accounts.8b00462. Epub 2018 Nov 21. Acc Chem Res. 2018. PMID: 30462481
-
Structures of liquid and aqueous water isotopologues at ambient temperature from ab initio path integral simulations.Phys Chem Chem Phys. 2022 May 11;24(18):10851-10859. doi: 10.1039/d2cp00499b. Phys Chem Chem Phys. 2022. PMID: 35504275
-
Erratum: Neutron Spin Echo Spectroscopy as a Unique Probe for Lipid Membrane Dynamics and Membrane-Protein Interactions.J Vis Exp. 2021 Aug 6;(174). doi: 10.3791/6475. J Vis Exp. 2021. PMID: 34358222
-
Opportunities and challenges for time-resolved studies of protein structural dynamics at X-ray free-electron lasers.Philos Trans R Soc Lond B Biol Sci. 2014 Jul 17;369(1647):20130318. doi: 10.1098/rstb.2013.0318. Philos Trans R Soc Lond B Biol Sci. 2014. PMID: 24914150 Free PMC article. Review.
References
-
- Draganić I. G., “ Radiolysis of water: A look at its origin and occurrence in the Nature,” Radiat. Phys. Chem. 72, 181 (2005).10.1016/j.radphyschem.2004.09.012 - DOI
-
- Sankari A., Stråhlman C., Sankari R., Partanen L., Laksman J., Kettunen J. A., Galván I. F., Lindh R., Malmqvist P.-Å., and Sorensen S. L., “ Non-radiative decay and fragmentation in water molecules after 1a−1 1 4a1 excitation and core ionization studied by electron-energy- resolved electron-ion coincidence spectroscopy,” J. Chem. Phys. 152, 074302 (2020).10.1063/1.5141414 - DOI - PubMed
-
- Piancastelli M. N., Hempelmann A., Heiser F., Gessner O., Rüdel A., and Becker U., “ Resonant photofragmentation of water at the oxygen K edge by high-resolution ion-yield spectroscopy,” Phys. Rev. A 59, 300 (1999).10.1103/PhysRevA.59.300 - DOI

