Immunophenotyping characteristics and outcome of COVID-19 patients: peripheral blood CD8+T cell as a prognostic biomarker for patients with Nirmatrelvir
- PMID: 37799722
- PMCID: PMC10547864
- DOI: 10.3389/fimmu.2023.1227905
Immunophenotyping characteristics and outcome of COVID-19 patients: peripheral blood CD8+T cell as a prognostic biomarker for patients with Nirmatrelvir
Abstract
Background: Nirmatrelvir has been authorized for the treatment of both hospitalized and non-hospitalized COVID-19 patients. However, the association between T lymphocyte subsets and the outcome of hospitalized COVID-19 patients treated with oral Nirmatrelvir has not been investigated. The objective of this study was to examine whether lymphocyte subsets could serve as biomarkers to assess the risk of mortality in COVID-19 patients undergoing Nirmatrelvir treatment, with the aim of enhancing medication management for COVID-19 patients.
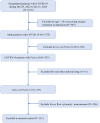
Methods: We conducted a retrospective cohort study at the Xiangya Hospital of Central South University in China between December 5, 2022 and January 31, 2023. The study reported demographic, clinical, T lymphocyte subsets, and inflammatory cytokine data of COVID-19 patients. We evaluated the associations of T lymphocyte subsets on admission with the composite outcome or death of patients using univariate and multivariable Cox regression analyses with hazards ratios (HRs) and 95% confidence intervals (CIs).
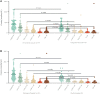
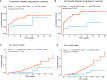
Results: We identified 2118 hospitalized COVID-19 patients during the study period, and conducted a follow-up of up to 38 days. Of these, 131 patients received Nirmatrelvir, with 56 (42.7%) in the composite outcome group, and 75 (57.3%) in the non-composite outcome group. Additionally, 101 (77.1%) patients were discharged, while 30 (22.9%) died. Our results showed a significant decrease in the CD3+, CD4+, and CD8+ T cell counts of patients in the composite outcome group and mortality group compared to the non-composite outcome group and discharged group, respectively. Multivariate Cox regression analysis showed that the significant decrease in CD8+ T cell count in peripheral blood was independently associated with the composite outcome in COVID-19 patients treated with Nirmatrelvir, with an HR of 1.96 (95%CI: 1.01-3.80). The significant decrease in CD4+ and CD8+ T cell counts in peripheral blood increased the hazard of developing mortality, with HRs of 6.48 (95%CI: 1.47-28.63) and 3.75 (95%CI: 1.27-11.11), respectively.
Conclusion: Our study revealed a significant positive correlation between a decrease in CD8+ T cell counts and progression and mortality of hospitalized COVID-19 patients treated with Nirmatrelvir. Lower counts (/μL) of CD8+ T cell (<201) were associated with a higher risk of in-hospital severity and death. Our findings may provide valuable references for physicians in optimizing the use of Nirmatrelvir.
Keywords: CD8+ T cell; COVID-19; T cell; biomarker; lymphocyte.
Copyright © 2023 Sun, Dian, Gao and Deng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

