Targeting BMI-1 to deplete antibody-secreting cells in autoimmunity
- PMID: 37799772
- PMCID: PMC10550498
- DOI: 10.1002/cti2.1470
Targeting BMI-1 to deplete antibody-secreting cells in autoimmunity
Abstract
Objectives: B cells drive the production of autoreactive antibody-secreting cells (ASCs) in autoimmune diseases such as Systemic Lupus Erythematosus (SLE) and Sjögren's syndrome, causing long-term organ damage. Current treatments for antibody-mediated autoimmune diseases target B cells or broadly suppress the immune system. However, pre-existing long-lived ASCs are often refractory to treatment, leaving a reservoir of autoreactive cells that continue to produce antibodies. Therefore, the development of novel treatment methods targeting ASCs is vital to improve patient outcomes. Our objective was to test whether targeting the epigenetic regulator BMI-1 could deplete ASCs in autoimmune conditions in vivo and in vitro.
Methods: Use of a BMI-1 inhibitor in both mouse and human autoimmune settings was investigated. Lyn -/- mice, a model of SLE, were treated with the BMI-1 small molecule inhibitor PTC-028, before assessment of ASCs, serum antibody and immune complexes. To examine human ASC survival, a novel human fibroblast-based assay was established, and the impact of PTC-028 on ASCs derived from Sjögren's syndrome patients was evaluated.
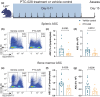
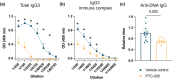
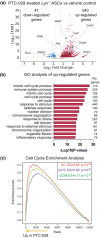
Results: BMI-1 inhibition significantly decreased splenic and bone marrow ASCs in Lyn -/- mice. The decline in ASCs was linked to aberrant cell cycle gene expression and led to a significant decrease in serum IgG3, immune complexes and anti-DNA IgG. PTC-028 was also efficacious in reducing ex vivo plasma cell survival from both Sjögren's syndrome patients and age-matched healthy donors.
Conclusion: These data provide evidence that inhibiting BMI-1 can deplete ASC in a variety of contexts and thus BMI-1 is a viable therapeutic target for antibody-mediated autoimmune diseases.
Keywords: B cells; BMI‐1; Sjögren's syndrome; Systemic Lupus Erythematosus; antibody; autoimmunity.
© 2023 The Authors. Clinical & Translational Immunology published by John Wiley & Sons Australia, Ltd on behalf of Australian and New Zealand Society for Immunology, Inc.
Conflict of interest statement
This study was supported in part by a GSK Fast Track Discovery Grant to KLG‐J. MR has received research funding for autoimmunity‐related clinical trials from BMS, Novartis, Servier Amgen and Astra Zeneca.
Figures

References
-
- Mielle J, Tison A, Cornec D, Le Pottier L, Daien C, Pers JO. B cells in Sjogren's syndrome: From pathophysiology to therapeutic target. Rheumatology (Oxford) 2021; 60: 2545–2560. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
