Evaluation of extracellular polymeric substances matrix volume, surface roughness and bacterial adhesion property of oral biofilm
- PMID: 37799886
- PMCID: PMC10547949
- DOI: 10.1016/j.jds.2022.12.022
Evaluation of extracellular polymeric substances matrix volume, surface roughness and bacterial adhesion property of oral biofilm
Abstract
Background/purpose: Oral biofilms are highly structured bacterial colonies embedded in a highly hydrated extracellular polymeric substances (EPS) matrix. This study aimed to investigate the characteristics of oral biofilm at different stages of maturation.
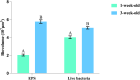
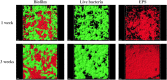
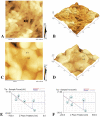
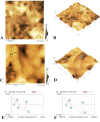
Materials and methods: Oral multispecies biofilms were grown anaerobically from plaque bacteria on collagen coated hydroxyapatite discs in brain heart infusion broth for one and three weeks. The volume of live bacteria and EPS matrix of the biofilms were determined by using corresponding fluorescent probes and confocal laser scanning microscopy. Atomic force microscopy (AFM) was used to quantitatively probe and correlate cell surface adhesion force of biofilms. The surface roughness was quantified in terms of the root mean square average of the height deviations. Adhesion was measured from force-distance data for the retraction of the cell from the surface.
Results: The volume of live bacteria and EPS of 3-week-old biofilms was higher than 1-week-old biofilms. The surface roughness value in 1-week-old biofilms was significantly higher than that in 3-week-old biofilms. AFM force-distance curve results showed that the adhesion force at the cell-cell interface was significantly more at-tractive than those at bacterial cells surface of both stages biofilms. Adhesion forces between the AFM tip and the surface of bacterial cell were fairly constant, whereas the cell-cell interface experienced greater adhesion forces in the biofilm's development.
Conclusion: As oral biofilms become mature, EPS volume and cell-cell adhesion forces increase while the surface roughness decreases.
Keywords: Atomic force microscopy; Bacterial adhesion; Biofilm; Extra-cellular polymeric substances; Surface roughness.
© 2023 Association for Dental Sciences of the Republic of China. Publishing services by Elsevier B.V.
Conflict of interest statement
The authors have no conflicts of interest relevant to this article.
Figures

Similar articles
-
In-situ, time-lapse study of extracellular polymeric substance discharge in Streptococcus mutans biofilm.Colloids Surf B Biointerfaces. 2017 Feb 1;150:98-105. doi: 10.1016/j.colsurfb.2016.11.031. Epub 2016 Nov 24. Colloids Surf B Biointerfaces. 2017. PMID: 27907861
-
The dynamics of bacterial proliferation, viability, and extracellular polymeric substances in oral biofilm development.J Dent. 2024 Apr;143:104882. doi: 10.1016/j.jdent.2024.104882. Epub 2024 Feb 6. J Dent. 2024. PMID: 38331378
-
Emergent Properties in Streptococcus mutans Biofilms Are Controlled through Adhesion Force Sensing by Initial Colonizers.mBio. 2019 Sep 10;10(5):e01908-19. doi: 10.1128/mBio.01908-19. mBio. 2019. PMID: 31506311 Free PMC article.
-
Physico-chemistry of bacterial transmission versus adhesion.Adv Colloid Interface Sci. 2017 Dec;250:15-24. doi: 10.1016/j.cis.2017.11.002. Epub 2017 Nov 5. Adv Colloid Interface Sci. 2017. PMID: 29129313 Review.
-
Physico-chemistry from initial bacterial adhesion to surface-programmed biofilm growth.Adv Colloid Interface Sci. 2018 Nov;261:1-14. doi: 10.1016/j.cis.2018.10.005. Epub 2018 Oct 24. Adv Colloid Interface Sci. 2018. PMID: 30376953 Review.
Cited by
-
The Influence of Nickel-Titanium (Ni-Ti) Rotary Instrument Systems on Debris and Smear Layer Formation in Endodontic Procedures: An In Vitro Scanning Electron Microscopy Study.Cureus. 2024 Feb 16;16(2):e54310. doi: 10.7759/cureus.54310. eCollection 2024 Feb. Cureus. 2024. PMID: 38496119 Free PMC article.
-
Endodontic Retreatment of a Mandibular Second Molar With a C-shaped Root Canal Configuration: A Case Report.Cureus. 2024 Jan 23;16(1):e52812. doi: 10.7759/cureus.52812. eCollection 2024 Jan. Cureus. 2024. PMID: 38389597 Free PMC article.
-
Comparative Evaluation of Bacterial Reduction by Laser-Activated Irrigation Technique (LAI) With Conventional Needle Irrigation (CNI) in Single-Rooted Teeth With Pulpal Necrosis: A Single-Blinded Randomized Controlled Trial.Cureus. 2023 Dec 17;15(12):e50666. doi: 10.7759/cureus.50666. eCollection 2023 Dec. Cureus. 2023. PMID: 38229788 Free PMC article.
-
Cleaning and Disinfecting Oval-Shaped Root Canals: Ex Vivo Evaluation of Three Rotary Instrumentation Systems with Passive Ultrasonic Irrigation.Medicina (Kaunas). 2023 May 16;59(5):962. doi: 10.3390/medicina59050962. Medicina (Kaunas). 2023. PMID: 37241194 Free PMC article.
-
Endodontic Treatment of a Mandibular Second Molar Featuring Vertucci Type V Configuration in the Distal Root: A Case Report.Cureus. 2024 May 8;16(5):e59905. doi: 10.7759/cureus.59905. eCollection 2024 May. Cureus. 2024. PMID: 38854290 Free PMC article.
References
-
- Iloche S., Wong L., Sissons C.H. Oral biofilms: emerging concepts in microbial ecology. J Dent Res. 2010;89:8–18. - PubMed
-
- Mosaddad S.A., Tahmasebi E., Yazdanian A., et al. Oral microbial biofilms: an update. Eur J Clin Microbiol Infect Dis. 2019;38:2005–2019. - PubMed
-
- Yang Y., Xia L., Haapasalo M., et al. A novel hydroxyapatite-binding antimicrobial peptide against oral biofilms. Clin Oral Invest. 2019;23:2705–2712. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

