The Durability of Antibody Responses of Two Doses of High-Dose Relative to Two Doses of Standard-Dose Inactivated Influenza Vaccine in Pediatric Hematopoietic Cell Transplant Recipients: A Multi-Center Randomized Controlled Trial
- PMID: 37800415
- PMCID: PMC10810702
- DOI: 10.1093/cid/ciad534
The Durability of Antibody Responses of Two Doses of High-Dose Relative to Two Doses of Standard-Dose Inactivated Influenza Vaccine in Pediatric Hematopoietic Cell Transplant Recipients: A Multi-Center Randomized Controlled Trial
Abstract
Background: Our previous study established a 2-dose regimen of high-dose trivalent influenza vaccine (HD-TIV) to be immunogenically superior compared to a 2-dose regimen of standard-dose quadrivalent influenza vaccine (SD-QIV) in pediatric allogeneic hematopoietic cell transplant (HCT) recipients. However, the durability of immunogenicity and the role of time post-HCT at immunization as an effect modifier are unknown.
Methods: This phase II, multi-center, double-blinded, randomized controlled trial compared HD-TIV to SD-QIV in children 3-17 years old who were 3-35 months post-allogeneic HCT, with each formulation administered twice, 28-42 days apart. Hemagglutination inhibition (HAI) titers were measured at baseline, 28-42 days following each dose, and 138-222 days after the second dose. Using linear mixed effects models, we estimated adjusted geometric mean HAI titer ratios (aGMR: HD-TIV/SD-QIV) to influenza antigens. Early and late periods were defined as 3-5 and 6-35 months post-HCT, respectively.
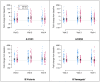
Results: During 3 influenza seasons (2016-2019), 170 participants were randomized to receive HD-TIV (n = 85) or SD-QIV (n = 85). HAI titers maintained significant elevations above baseline for both vaccine formulations, although the relative immunogenic benefit of HD-TIV to SD-QIV waned during the study. A 2-dose series of HD-TIV administered late post-HCT was associated with higher GMTs compared to the early post-HCT period (late group: A/H1N1 aGMR = 2.16, 95% confidence interval [CI] = [1.14-4.08]; A/H3N2 aGMR = 3.20, 95% CI = [1.60-6.39]; B/Victoria aGMR = 1.91, 95% CI = [1.01-3.60]; early group: A/H1N1 aGMR = 1.03, 95% CI = [0.59-1.80]; A/H3N2 aGMR = 1.23, 95% CI = [0.68-2.25]; B/Victoria aGMR = 1.06, 95% CI = [0.56-2.03]).
Conclusions: Two doses of HD-TIV were more immunogenic than SD-QIV, especially when administered ≥6 months post-HCT. Both groups maintained higher titers compared to baseline throughout the season.
Clinical trials registration: NCT02860039.
Keywords: high dose; influenza; pediatrics; stem cell recipients; vaccination.
© The Author(s) 2023. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest . N. B. H. received grant support from Sanofi and Quidel and a current grant from Merck. C. E. B. receives grant support from Pfizer and Merck. J. A. E. reports grant support from AstraZeneca, Merck, Pfizer, and GlaxoSmithKline (GSK) (all paid to institution); consultant fees for Abbvie, AstraZeneca, Meissa Vaccines, Moderna, Pfizer, Sanofi Pasteur, Shinogi, and Ark Biopharma. G. M. reports Research support from Astellas Inc and SymBio Pharmaceuticals. C. L. K. reports payment for partipcation on an advisory board for Horizon Therapeutics; payment for educational lectures from Medscape, i3Health, Physicians Education Resource, and Dava Oncology; travel support for global summit on Hematologic Malignancies from Dava Oncology; and payment from CSL Behring for providing graft versus host disease (GVHD) adjudication for a clinical trial. M. I. A. reports institutional research grants from Miravista Dianostics and Merck; consulting fees from Karius; travel support for meeting attendance from Pediatric Infectious Diseases Society and American Academy of Pediatrics; and an unpaid position on Pediatric Infectious Diseases Society Board of Directors. C. J. H. reports grants or contracts from GSK, Pfizer, Merck, and Astellas; royalties from UpToDate; payment or honoraria for educational material from Pediatric News; and non-financial interests in the American Board of Pediatrics. J. L. F. reports consulting fees and stock options from Massive Bio, Inc. J. E. S. reports institutional grants or contracts from the Centers for Disease Control and Prevention (CDC), National Institutes of Health (NIH), and Food and Drug Administration (FDA); personal consulting fees from Association of Professionals in Infection Control and Epidemiology and Association of American Medical Colleges; and honoraria for speaking engagements from Missouri American Academy of Pediatrics. E. A. M. reports institutional grants or contracts for clinical trials of pediatric coronavirus disease (COVID) vaccines and therapeutics from Pfizer. G. C. P. reports institutional grants or contracts for coronavirus disease 2019 (COVID-19) clinical trials from Pfizer and Moderna, Inc. F. M. M. reports grants or contracts paid to institution from Gilead for antiviral research and from Pfizer for vaccine research, and grant support from NIH and CDC; participation as DSMB member, payments to author, for Pfizer and Moderna. A. J. S. reports grant support from NIH. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures

References
-
- Nichols WG, Guthrie KA, Corey L, Boeckh M. Influenza infections after hematopoietic stem cell transplantation: risk factors, mortality, and the effect of antiviral therapy. Clin Infect Dis 2004; 39:1300–6. - PubMed
-
- Luján-Zilbermann J, Benaim E, Tong X, Srivastava DK, Patrick CC, DeVincenzo JP. Respiratory virus infections in pediatric hematopoietic stem cell transplantation. Clin Infect Dis 2001; 33:962–8. - PubMed
-
- Shang M, Blanton L, Brammer L, Olsen SJ, Fry AM. Influenza-associated pediatric deaths in the United States, 2010–2016. Pediatrics 2018; 141:e20172918. - PubMed

