Comparison of efficacy and safety between neoadjuvant chemotherapy and neoadjuvant immune checkpoint inhibitors combined with chemotherapy for locally advanced esophageal squamous cell carcinoma: a systematic review and meta-analysis
- PMID: 37800587
- PMCID: PMC10793745
- DOI: 10.1097/JS9.0000000000000816
Comparison of efficacy and safety between neoadjuvant chemotherapy and neoadjuvant immune checkpoint inhibitors combined with chemotherapy for locally advanced esophageal squamous cell carcinoma: a systematic review and meta-analysis
Abstract
Background: The application of neoadjuvant immune checkpoint inhibitors combined with chemotherapy (NICT) in treating locally advanced oesophageal squamous cell carcinoma (ESCC) is a subject of considerable research interest. In light of this, we undertook a comprehensive meta-analysis aiming to compare the efficacy and safety of this novel approach with conventional neoadjuvant chemotherapy (NCT) in the management of ESCC.
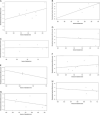
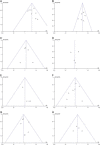
Methods: A systematic search was conducted in PubMed, Embase, Cochrane Library, and Web of Science to gather relevant literature on the efficacy and safety of NICT compared to conventional NCT in locally advanced ESCC published before June 2023. Effect indicators, including odds ratios (ORs) with associated 95% CIs, were employed to evaluate the safety and efficacy outcomes. The risk of bias was assessed using the Cochrane bias risk assessment tool, and s ubgroup analysis and sensitivity analysis were conducted to investigate the findings further.
Results: A total of nine studies qualified for the meta-analysis, all of which investigated the efficacy and safety of NICT compared to conventional NCT. The pooled rates of pathologic complete response and major pathologic response in the NICT group were significantly higher compared to the NCT group, with values of 26.9% versus 8.3% ( P <0.00001) and 48.1% versus 24.6% ( P <0.00001), respectively. The ORs for achieving pathologic complete response and major pathologic response were 4.24 (95% CI, 2.84-6.32, I 2 =14%) and 3.30 (95% CI, 2.31-4.71, I 2 =0%), respectively, indicating a significant advantage for the NICT group. Regarding safety outcomes, the pooled incidences of treatment-related adverse events and serious adverse events in the NICT group were 64.4% and 11.5%, respectively, compared to 73.8% and 9.3% in the NCT group. However, there were no significant differences observed between the two groups in terms of treatment-related adverse events (OR=0.67, 95% CI, 0.29-1.54, P =0.35, I 2 =58%) or serious adverse events (OR=1.28, 95% CI, 0.69-2.36, P =0.43, I 2 =0%). Furthermore, no significant differences were found between the NICT and NCT groups regarding R0 resection rates, anastomotic leakage, pulmonary infection, and postoperative hoarseness.
Conclusions: Neoadjuvant immune checkpoint inhibitors combined with chemotherapy demonstrate efficacy and safety in treating resectable oesophageal squamous cell carcinoma. Nevertheless, additional randomized trials are required to confirm the optimal treatment regimen.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
No conflict of interest.
Figures







Similar articles
-
Efficacy and Safety of Neoadjuvant Chemotherapy with or without Immune Checkpoint Inhibitors for Resectable Esophageal Squamous Cell Carcinoma: a Meta-analysis of Randomized Controlled Trials.J Gastrointest Cancer. 2025 Aug 11;56(1):172. doi: 10.1007/s12029-025-01273-1. J Gastrointest Cancer. 2025. PMID: 40788432 Review.
-
Clinical efficacy of different neoadjuvant therapies for resectable esophageal squamous cell carcinoma.World J Surg Oncol. 2025 Jun 20;23(1):243. doi: 10.1186/s12957-025-03897-w. World J Surg Oncol. 2025. PMID: 40542352 Free PMC article.
-
Survival Benefits of Neoadjuvant Immunochemotherapy Versus Chemotherapy in Locally Advanced Esophageal Squamous Cell Carcinoma: A Propensity Matched Score.J Gastrointest Cancer. 2025 Aug 4;56(1):167. doi: 10.1007/s12029-025-01293-x. J Gastrointest Cancer. 2025. PMID: 40760358
-
The efficacy and safety of neoadjuvant immunotherapy in resectable locally advanced esophageal squamous cell carcinoma: A systematic review and meta-analysis.Front Immunol. 2023 Feb 17;14:1118902. doi: 10.3389/fimmu.2023.1118902. eCollection 2023. Front Immunol. 2023. PMID: 36875107 Free PMC article.
-
Analysis of the safety and efficacy of PD-1/PD-L1 inhibitors combined with chemotherapy in the treatment of locally advanced resectable esophageal squamous cell carcinoma: a systematic review and meta-analysis based on four randomized controlled trials.Front Oncol. 2025 Aug 13;15:1590111. doi: 10.3389/fonc.2025.1590111. eCollection 2025. Front Oncol. 2025. PMID: 40881857 Free PMC article.
Cited by
-
A commentary on 'Comparison of efficacy and safety between neoadjuvant chemotherapy and neoadjuvant immune checkpoint inhibitors combined with chemotherapy for locally advanced esophageal squamous cell carcinoma: a systematic review and meta-analysis'.Int J Surg. 2024 May 1;110(5):3118-3119. doi: 10.1097/JS9.0000000000001211. Int J Surg. 2024. PMID: 38363989 Free PMC article. No abstract available.
-
Enhancing survival in locally advanced esophageal cancer: a comparative analysis of neoadjuvant immunotherapy versus conventional neoadjuvant therapies using the SEER database.J Thorac Dis. 2025 May 30;17(5):2778-2801. doi: 10.21037/jtd-24-1905. Epub 2025 May 28. J Thorac Dis. 2025. PMID: 40529733 Free PMC article.
-
Current status and perspectives of esophageal cancer: a comprehensive review.Cancer Commun (Lond). 2025 Mar;45(3):281-331. doi: 10.1002/cac2.12645. Epub 2024 Dec 26. Cancer Commun (Lond). 2025. PMID: 39723635 Free PMC article. Review.
-
Colorectal anastomotic leakage after conversion surgery for advanced endometrial cancer treated with lenvatinib plus pembrolizumab: a case report.Int Cancer Conf J. 2024 Dec 10;14(1):64-71. doi: 10.1007/s13691-024-00739-6. eCollection 2025 Jan. Int Cancer Conf J. 2024. PMID: 39758791 Free PMC article.
-
Efficacy and safety of neoadjuvant immunotherapy combined with chemotherapy in patients with resectable esophageal squamous cell carcinoma: a systematic review and meta-analysis.Transl Cancer Res. 2025 Jun 30;14(6):3373-3399. doi: 10.21037/tcr-2024-2631. Epub 2025 Jun 18. Transl Cancer Res. 2025. PMID: 40687234 Free PMC article.
References
-
- Sung H, Ferlay J, Siegel RL, et al. . Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–249. - PubMed
-
- Arnold M, Soerjomataram I, Ferlay J, et al. . Global incidence of oesophageal cancer by histological subtype in 2012. Gut 2015;64:381–387. - PubMed
-
- Baba NYY, Kinoshita K, Iwatsuki M, et al. . And prognostic features of patients with esophageal cancer and multiple primary cancers: a retrospective single-institution study. Ann Surg 2018;267:478–83. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

