Inflammatory signaling pathways in the treatment of Alzheimer's disease with inhibitors, natural products and metabolites (Review)
- PMID: 37800614
- PMCID: PMC10558228
- DOI: 10.3892/ijmm.2023.5314
Inflammatory signaling pathways in the treatment of Alzheimer's disease with inhibitors, natural products and metabolites (Review)
Abstract
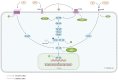
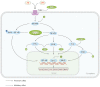
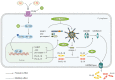
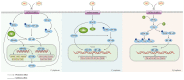
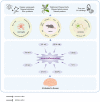
The intricate nature of Alzheimer's disease (AD) pathogenesis poses a persistent obstacle to drug development. In recent times, neuroinflammation has emerged as a crucial pathogenic mechanism of AD, and the targeting of inflammation has become a viable approach for the prevention and management of AD. The present study conducted a comprehensive review of the literature between October 2012 and October 2022, identifying a total of 96 references, encompassing 91 distinct pharmaceuticals that have been investigated for their potential impact on AD by inhibiting neuroinflammation. Research has shown that pharmaceuticals have the potential to ameliorate AD by reducing neuroinflammation mainly through regulating inflammatory signaling pathways such as NF‑κB, MAPK, NLRP3, PPARs, STAT3, CREB, PI3K/Akt, Nrf2 and their respective signaling pathways. Among them, tanshinone IIA has been extensively studied for its anti‑inflammatory effects, which have shown significant pharmacological properties and can be applied clinically. Thus, it may hold promise as an effective drug for the treatment of AD. The present review elucidated the inflammatory signaling pathways of pharmaceuticals that have been investigated for their therapeutic efficacy in AD and elucidates their underlying mechanisms. This underscores the auspicious potential of pharmaceuticals in ameliorating AD by impeding neuroinflammation.
Keywords: Alzheimer's disease; inflammatory signaling pathways; inhibitor; neuroinflammation; treatment.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Inflammatory signaling pathways in Alzheimer's disease: Mechanistic insights and possible therapeutic interventions.Ageing Res Rev. 2025 Feb;104:102548. doi: 10.1016/j.arr.2024.102548. Epub 2024 Oct 16. Ageing Res Rev. 2025. PMID: 39419399 Review.
-
P-coumaric acid ameliorates Aβ25-35-induced brain damage in mice by modulating gut microbiota and serum metabolites.Biomed Pharmacother. 2023 Dec;168:115825. doi: 10.1016/j.biopha.2023.115825. Epub 2023 Nov 2. Biomed Pharmacother. 2023. PMID: 37924791
-
Myricetin improves pathological changes in 3×Tg-AD mice by regulating the mitochondria-NLRP3 inflammasome-microglia channel by targeting P38 MAPK signaling pathway.Phytomedicine. 2023 Jul;115:154801. doi: 10.1016/j.phymed.2023.154801. Epub 2023 Apr 6. Phytomedicine. 2023. PMID: 37086707
-
Anti-Neuroinflammatory Potential of Natural Products in the Treatment of Alzheimer's Disease.Molecules. 2023 Feb 3;28(3):1486. doi: 10.3390/molecules28031486. Molecules. 2023. PMID: 36771152 Free PMC article. Review.
-
Implications of Phosphoinositide 3-Kinase-Akt (PI3K-Akt) Pathway in the Pathogenesis of Alzheimer's Disease.Mol Neurobiol. 2022 Jan;59(1):354-385. doi: 10.1007/s12035-021-02611-7. Epub 2021 Oct 26. Mol Neurobiol. 2022. PMID: 34699027 Review.
Cited by
-
Neuroinflammation and Neurodegenerative Diseases: How Much Do We Still Not Know?Brain Sci. 2023 Dec 23;14(1):19. doi: 10.3390/brainsci14010019. Brain Sci. 2023. PMID: 38248234 Free PMC article. Review.
-
Therapeutic potential of luteolin in neurodegenerative disorders: targeting Nrf2, NFĸB, MAPK, and JAK-STAT pathways to combat neuroinflammation and apoptosis.Inflammopharmacology. 2025 Jul 22. doi: 10.1007/s10787-025-01846-3. Online ahead of print. Inflammopharmacology. 2025. PMID: 40694206 Review.
-
Recent advances in Alzheimer's disease: Mechanisms, clinical trials and new drug development strategies.Signal Transduct Target Ther. 2024 Aug 23;9(1):211. doi: 10.1038/s41392-024-01911-3. Signal Transduct Target Ther. 2024. PMID: 39174535 Free PMC article. Review.
-
The effect of tanshinones on cognitive impairments in animal models of Alzheimer's disease: a systematic review and meta-analysis.Front Pharmacol. 2025 Feb 27;16:1529327. doi: 10.3389/fphar.2025.1529327. eCollection 2025. Front Pharmacol. 2025. PMID: 40083386 Free PMC article.
-
Spatiotemporal Dysregulation of Neuron-Glia Related Genes and Pro-/Anti-Inflammatory miRNAs in the 5xFAD Mouse Model of Alzheimer's Disease.Int J Mol Sci. 2024 Aug 31;25(17):9475. doi: 10.3390/ijms25179475. Int J Mol Sci. 2024. PMID: 39273422 Free PMC article.
References
-
- Wang X, Iyaswamy A, Xu D, Krishnamoorthi S, Sreenivasmurthy SG, Yang Y, Li Y, Chen C, Li M, Li HW, Wong MS. Real-time detection and visualization of amyloid-β aggregates induced by hydrogen peroxide in cell and mouse models of Alzheimer's disease. ACS Appl Mater Interfaces. 2023;15:39–47. doi: 10.1021/acsami.2c07859. - DOI - PMC - PubMed
-
- Nasaruddin ML, Pan X, McGuinness B, Passmore P, Kehoe PG, Holscher C, Graham SF, Green BD. Evidence that parietal lobe fatty acids may be more profoundly affected in moderate Alzheimer's disease (AD) pathology than in severe AD pathology. Metabolites. 2018;8:69. doi: 10.3390/metabo8040069. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

