Mycobacteriophage D29 Lysin B exhibits promising anti-mycobacterial activity against drug-resistant Mycobacterium tuberculosis
- PMID: 37800970
- PMCID: PMC10714809
- DOI: 10.1128/spectrum.04597-22
Mycobacteriophage D29 Lysin B exhibits promising anti-mycobacterial activity against drug-resistant Mycobacterium tuberculosis
Abstract
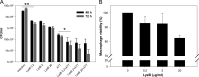
To combat the rapidly emerging drug-resistant M. tuberculosis, it is now essential to look for alternative therapeutics. Mycobacteriophages can be considered as efficient therapeutics due to their natural ability to infect and kill mycobacteria including M. tuberculosis. Here, we have exploited the mycolyl-arabinogalactan esterase property of LysB encoded from mycobacteriophage D29. This study is novel in terms of targeting a multi-drug-resistant pathogenic strain of M. tuberculosis with LysB and also examining the combination of anti-TB drugs and LysB. All the experiments include external administration of LysB. Therefore, the remarkable lytic activity of LysB overcomes the difficulty to enter the complex cell envelope of mycobacteria. Targeting the intracellularly located M. tuberculosis by LysB and non-toxicity to macrophages take the process of the development of LysB as a drug one step ahead, and also, the interaction studies with rifampicin and isoniazid will help to form a new treatment regimen against tuberculosis.
Keywords: D29 mycobacteriophage; additive effect; antibiotic resistance; mycolyl-arabinogalactan esterase; phage therapy; tuberculosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- WHO . 2021. Fact sheet details tuberculosis.
-
- Chakaya J, Khan M, Ntoumi F, Aklillu E, Fatima R, Mwaba P, Kapata N, Mfinanga S, Hasnain SE, Katoto P, Bulabula ANH, Sam-Agudu NA, Nachega JB, Tiberi S, McHugh TD, Abubakar I, Zumla A. 2021. Global tuberculosis report 2020 - reflections on the global TB burden, treatment and prevention efforts. Int J Infect Dis 113:S7–S12. doi:10.1016/j.ijid.2021.02.107 - DOI - PMC - PubMed
-
- (WHO) WHO. 2017. Guidelines for treatment of drug-susceptible tuberculosis and patient care. Available from: https://appswhoint/iris/bitstream/handle/10665/255052/9789241550000-engpdf
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

