Evaluation of in vitro antiviral activity of SARS-CoV-2 Mpro inhibitor pomotrelvir and cross-resistance to nirmatrelvir resistance substitutions
- PMID: 37800975
- PMCID: PMC10649086
- DOI: 10.1128/aac.00840-23
Evaluation of in vitro antiviral activity of SARS-CoV-2 Mpro inhibitor pomotrelvir and cross-resistance to nirmatrelvir resistance substitutions
Abstract
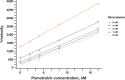
The unprecedented scale of the COVID-19 pandemic and the rapid evolution of SARS-CoV-2 variants underscore the need for broadly active inhibitors with a high barrier to resistance. The coronavirus main protease (Mpro) is an essential cysteine protease required for viral polyprotein processing and is highly conserved across human coronaviruses. Pomotrelvir is a novel Mpro inhibitor that has recently completed a phase 2 clinical trial. In this report, we demonstrated that pomotrelvir is a potent competitive inhibitor of SARS-CoV-2 Mpro with high selectivity against human proteases. In the enzyme assay, pomotrelvir is also active against Mpro proteins derived from human coronaviruses CoV-229E, CoV-OC43, CoV-HKU1, CoV-NL63, MERS, and SARS-CoV. In cell-based SARS-CoV-2 replicon and SARS-CoV-2 infection assays, pomotrelvir has shown potent inhibitory activity and is broadly active against SARS-CoV-2 clinical isolates including Omicron variants. Many resistance substitutions of the Mpro inhibitor nirmatrelvir confer cross-resistance to pomotrelvir, consistent with the finding from our enzymatic analysis that pomotrelvir and nirmatrelvir compete for the same binding site. In a SARS-CoV-2 infection assay, pomotrelvir is additive when combined with remdesivir or molnupiravir, two nucleoside analogs targeting viral RNA synthesis. In conclusion, our results from the in vitro characterization of pomotrelvir antiviral activity support its further clinical development as an alternative COVID-19 therapeutic option.
Keywords: Mpro inhibitor; PBI-0451; SARS-CoV-2; antiviral resistance; coronavirus; pomotrelvir.
Conflict of interest statement
All employees of Pardes Biosciences were granted stock options in the company.
Figures


References
-
- Dhama K, Patel SK, Sharun K, Pathak M, Tiwari R, Yatoo MI, Malik YS, Sah R, Rabaan AA, Panwar PK, Singh KP, Michalak I, Chaicumpa W, Martinez-Pulgarin DF, Bonilla-Aldana DK, Rodriguez-Morales AJ. 2020. SARS-CoV-2 jumping the species barrier: zoonotic lessons from SARS, MERS and recent advances to combat this pandemic virus. Travel Med Infect Dis 37:101830. doi:10.1016/j.tmaid.2020.101830 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

