Mechanisms by which the cystic fibrosis transmembrane conductance regulator may influence SARS-CoV-2 infection and COVID-19 disease severity
- PMID: 37801035
- PMCID: PMC10760435
- DOI: 10.1096/fj.202300077R
Mechanisms by which the cystic fibrosis transmembrane conductance regulator may influence SARS-CoV-2 infection and COVID-19 disease severity
Abstract
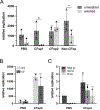
Patients with cystic fibrosis (CF) exhibit pronounced respiratory damage and were initially considered among those at highest risk for serious harm from SARS-CoV-2 infection. Numerous clinical studies have subsequently reported that individuals with CF in North America and Europe-while susceptible to severe COVID-19-are often spared from the highest levels of virus-associated mortality. To understand features that might influence COVID-19 among patients with cystic fibrosis, we studied relationships between SARS-CoV-2 and the gene responsible for CF (i.e., the cystic fibrosis transmembrane conductance regulator, CFTR). In contrast to previous reports, we found no association between CFTR carrier status (mutation heterozygosity) and more severe COVID-19 clinical outcomes. We did observe an unexpected trend toward higher mortality among control individuals compared with silent carriers of the common F508del CFTR variant-a finding that will require further study. We next performed experiments to test the influence of homozygous CFTR deficiency on viral propagation and showed that SARS-CoV-2 production in primary airway cells was not altered by the absence of functional CFTR using two independent protocols. On the contrary, experiments performed in vitro strongly indicated that virus proliferation depended on features of the mucosal fluid layer known to be disrupted by absent CFTR in patients with CF, including both low pH and increased viscosity. These results point to the acidic, viscous, and mucus-obstructed airways in patients with cystic fibrosis as unfavorable for the establishment of coronaviral infection. Our findings provide new and important information concerning relationships between the CF clinical phenotype and severity of COVID-19.
Keywords: COVID-19; SARS-CoV-2; comorbidity; cystic fibrosis transmembrane conductance regulator; mucus membrane; virus replication.
© 2023 Federation of American Societies for Experimental Biology.
Conflict of interest statement
Conflict of interest statement
The authors declare no competing interest.
Figures

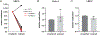
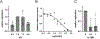
References
-
- CDC, People with Certain Medical Conditions. (May 13, 2021).
-
- CysticFibrosisFoundation (2021) COVID-19 [video]. (https://www.youtube.com/watch?v=8BV2JH2wRFM).
-
- Bustamante L. (2021) Cystic fibrosis patients fight for higher spot in coronavirus vaccine line [video]. (NBC10 Philadelphia, https://www.nbcphiladelphia.com/news/coronavirus/cystic-fibrosis-patient...).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

