Rapid Response to Remdesivir in Hospitalised COVID-19 Patients: A Propensity Score Weighted Multicentre Cohort Study
- PMID: 37801280
- PMCID: PMC10600071
- DOI: 10.1007/s40121-023-00874-2
Rapid Response to Remdesivir in Hospitalised COVID-19 Patients: A Propensity Score Weighted Multicentre Cohort Study
Abstract
Introduction: Remdesivir is a registered treatment for hospitalised patients with COVID-19 that has moderate clinical effectiveness. Anecdotally, some patients' respiratory insufficiency seemed to recover particularly rapidly after initiation of remdesivir. In this study, we investigated if this rapid improvement was caused by remdesivir, and which patient characteristics might predict a rapid clinical improvement in response to remdesivir.
Methods: This was a multicentre observational cohort study of hospitalised patients with COVID-19 who required supplemental oxygen and were treated with dexamethasone. Rapid clinical improvement in response to treatment was defined by a reduction of at least 1 L of supplemental oxygen per minute or discharge from the hospital within 72 h after admission. Inverse probability of treatment-weighted logistic regression modelling was used to assess the association between remdesivir and rapid clinical improvement. Secondary endpoints included in-hospital mortality, ICU admission rate and hospitalisation duration.
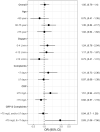
Results: Of 871 patients included, 445 were treated with remdesivir. There was no influence of remdesivir on the occurrence of rapid clinical improvement (62% vs 61% OR 1.05, 95% CI 0.79-1.40; p = 0.76). The in-hospital mortality was lower (14.7% vs 19.8% OR 0.70, 95% CI 0.48-1.02; p = 0.06) for the remdesivir-treated patients. Rapid clinical improvement occurred more often in patients with low C-reactive protein (≤ 75 mg/L) and short duration of symptoms prior to hospitalisation (< 7 days) (OR 2.84, 95% CI 1.07-7.56).
Conclusion: Remdesivir generally does not increase the incidence of rapid clinical improvement in hospitalised patients with COVID-19, but it might have an effect in patients with short duration of symptoms and limited signs of systemic inflammation.
Keywords: COVID-19; Hospitalized patients; Rapid clinical improvement; Remdesivir.
© 2023. The Author(s).
Conflict of interest statement
Emiel Leegwater and Erik B Wilms of The Hague Hospital Pharmacy received an unrestricted research grant from Gilead Sciences Inc. T.H.O. participated in a COVID-19 Digital Advisory Board from Gilead Sciences Inc. Lisa Dol, Menno R Benard, Eveline E Roelofsen, Nathalie M Delfos, Machteld van der Feltz, Femke PN Mollema, Liesbeth BE Bosma, Loes E Visser, Thomas H Ottens, Nathalie D van Burgel, Sesmu M Arbous, Lahssan H El Bouazzaoui, Rachel Knevel, Rolf HH Groenwold, Mark GJ de Boer, Leo G Visser, Frits R Rosendaal, and Cees van Nieuwkoop have nothing to disclose.
Figures
References
-
- Infectious Diseases Society of America. ISDA guidelines on the treatment and management of patients with COVID-19. Infectious Diseases Society of America. 2022. https://www.idsociety.org/practice-guideline/covid-19-guideline-treatmen.... Accessed 5 Jan 2023.
LinkOut - more resources
Full Text Sources
Research Materials