Fibroblast Activation Protein-Targeted Radioligand Therapy with 177Lu-EB-FAPI for Metastatic Radioiodine-Refractory Thyroid Cancer: First-in-Human, Dose-Escalation Study
- PMID: 37801296
- PMCID: PMC10690094
- DOI: 10.1158/1078-0432.CCR-23-1983
Fibroblast Activation Protein-Targeted Radioligand Therapy with 177Lu-EB-FAPI for Metastatic Radioiodine-Refractory Thyroid Cancer: First-in-Human, Dose-Escalation Study
Abstract
Purpose: Fibroblast activation protein (FAP) is a promising target for tumor treatment. In this study, we aimed to investigate the safety and efficacy of the albumin binder-conjugated FAP-targeted radiopharmaceutical, 177Lu-EB-FAPI (177Lu-LNC1004), in patients with metastatic radioiodine-refractory thyroid cancer (mRAIR-TC).
Patients and methods: This open-label, non-randomized, first-in-human, dose-escalation, investigator-initiated trial had a 3+3 design and involved a 6-week 177Lu-LNC1004 treatment cycle in patients with mRAIR-TC at 2.22 GBq initially, with subsequent cohorts receiving an incremental 50% dose increase until dose-limiting toxicity (DLT) was observed.
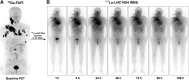
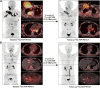
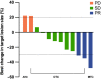
Results: 177Lu-LNC1004 administration was well tolerated, with no life-threatening adverse events observed. No patients experienced DLT in Group A (2.22 GBq/cycle). One patient experienced grade 4 thrombocytopenia in Group B (3.33 GBq/cycle); hence, another three patients were enrolled, none of whom experienced DLT. Two patients experienced grade 3 and 4 hematotoxicity in Group C (4.99 GBq/cycle). The mean whole-body effective dose was 0.17 ± 0.04 mSv/MBq. Intense 177Lu-LNC1004 uptake and prolonged tumor retention resulted in high mean absorbed tumor doses (8.50 ± 12.36 Gy/GBq). The mean effective half-lives for the whole-body and tumor lesions were 90.20 ± 7.68 and 92.46 ± 9.66 hours, respectively. According to RECIST, partial response, stable disease, and progressive disease were observed in 3 (25%), 7 (58%), and 2 (17%) patients, respectively. The objective response and disease control rates were 25% and 83%, respectively.
Conclusions: FAP-targeted radioligand therapy with 177Lu-LNC1004 at 3.33 GBq/cycle was well tolerated in patients with advanced mRAIR-TC, with high radiation dose delivery to the tumor lesions, encouraging therapeutic efficacy, and acceptable side effects.
©2023 The Authors; Published by the American Association for Cancer Research.
Figures

References
-
- Chen H, Zhao L, Ruan D, Pang Y, Hao B, Dai Y, et al. . Usefulness of [(68)Ga]Ga-DOTA-FAPI-04 PET/CT in patients presenting with inconclusive [(18)F]FDG PET/CT findings. Eur J Nucl Med Mol Imaging 2021;48:73–86. - PubMed
-
- Welt S, Divgi CR, Scott AM, Garin-Chesa P, Finn RD, Graham M, et al. . Antibody targeting in metastatic colon cancer: a phase I study of monoclonal antibody F19 against a cell-surface protein of reactive tumor stromal fibroblasts. J Clin Oncol 1994;12:1193–203. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 82102094/the National Natural Science Foundation of China
- 82071961/the National Natural Science Foundation of China
- 82272037/the National Natural Science Foundation of China
- 2022J05314/the Fujian Natural Science Foundation for Youth Innovation
- 2021ZQNZD016/the Fujian Research and Training Grants for Young and Middle aged Leaders in Healthcare, Key Scientific Research Program for Young Scholars in Fujian
- 2022D005/the Fujian Natural Science Foundation for Distinguished Young Scholars
- 3502Z20209269/the Xiamen Medical and Health Guidance Projects
- 3502Z20224ZD1001/the Xiamen Medical and Health Guidance Projects
- NUHSRO/2020/133/Startup/08/the National University of Singapore Start-up Grant
- NUHSRO/2021/097/Startup/13/the National University of Singapore Start-up Grant
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

