Confocal Raman Spectroscopic Characterization of Dermatopharmacokinetics Ex Vivo
- PMID: 37801410
- PMCID: PMC10630943
- DOI: 10.1021/acs.molpharmaceut.3c00755
Confocal Raman Spectroscopic Characterization of Dermatopharmacokinetics Ex Vivo
Abstract
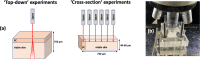
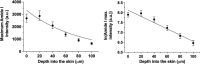
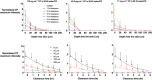
Confocal Raman spectroscopy is being assessed as a tool with which to quantify the rate and extent of drug uptake to and its clearance from target sites of action within the viable epidermis below the skin's stratum corneum (SC) barrier. The objective of this research was to confirm that Raman can interrogate drug disposition within the living layers of the skin (where many topical drugs elicit their pharmacological effects) and to identify procedures by which Raman signal attenuation with increasing skin depth may be corrected and normalized so that metrics descriptive of topical bioavailability may be identified. It was first shown in experiments on skin cross-sections parallel to the skin surface that the amide I signal, originating primarily from keratin, was quite constant with depth into the skin and could be used to correct for signal attenuation when confocal Raman data were acquired in a "top-down" fashion. Then, using 4-cyanophenol (CP) as a model skin penetrant with a strong Raman-active C≡N functionality, a series of uptake and clearance experiments, performed as a function of time, demonstrated clearly that normalized spectroscopic data were able to detect the penetrant to at least 40-80 μm into the skin and to distinguish the disposition of CP from different vehicles. Metrics related to local bioavailability (and potentially bioequivalence) included areas under the normalized C≡N signal versus depth profiles and elimination rate constants deduced post-removal of the formulations. Finally, Raman measurements were made with an approved dermatological drug, crisaborole, for which delivery from a fully saturated formulation into the skin layers just below the SC was detectable.
Keywords: Raman spectroscopy; skin clearance; skin penetration; skin uptake; topical bioavailability.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



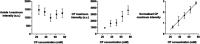

References
-
- Guidance for Industry: Bioavailability and Bioequivalence Studies Submitted in NDAs or INDs — General Considerations. https://www.fda.gov/files/drugs/published/Bioavailability-and-Bioequival... (accessed 17 Jan, 2023).
-
- Maciel Tabosa M. A.; Vitry P.; Zarmpi P.; Bunge A. L.; Belsey N. A.; Tsikritsis D.; Woodman T. J.; White K. J.; Delgado-Charro M. B.; Guy R. H. Quantification of Chemical Uptake into the Skin by Vibrational Spectroscopies and Stratum Corneum Sampling. Mol. Pharm. 2023, 20, 2527–2535. 10.1021/acs.molpharmaceut.2c01109. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

