Complement factor D targeting protects endotheliopathy in organoid and monkey models of COVID-19
- PMID: 37802037
- PMCID: PMC10575686
- DOI: 10.1016/j.stem.2023.09.001
Complement factor D targeting protects endotheliopathy in organoid and monkey models of COVID-19
Abstract
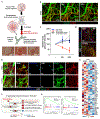
COVID-19 is linked to endotheliopathy and coagulopathy, which can result in multi-organ failure. The mechanisms causing endothelial damage due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) remain elusive. Here, we developed an infection-competent human vascular organoid from pluripotent stem cells for modeling endotheliopathy. Longitudinal serum proteome analysis identified aberrant complement signature in critically ill patients driven by the amplification cycle regulated by complement factor B and D (CFD). This deviant complement pattern initiates endothelial damage, neutrophil activation, and thrombosis specific to organoid-derived human blood vessels, as verified through intravital imaging. We examined a new long-acting, pH-sensitive (acid-switched) antibody targeting CFD. In both human and macaque COVID-19 models, this long-acting anti-CFD monoclonal antibody mitigated abnormal complement activation, protected endothelial cells, and curtailed the innate immune response post-viral exposure. Collectively, our findings suggest that the complement alternative pathway exacerbates endothelial injury and inflammation. This underscores the potential of CFD-targeted therapeutics against severe viral-induced inflammathrombotic outcomes.
Keywords: CFD; SARS-CoV-2; acid-switch half-life extended antibody; complement; cynomolgus macaque; endotheliopathy; iPSC; thrombopathy; vascular organoid.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests N.S. and T.T. are patent holders associated with the technology described in this project.
Figures
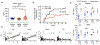



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

