High-dimensional analysis of T-cell profiling variations following belimumab treatment in systemic lupus erythematosus
- PMID: 37802602
- PMCID: PMC10565340
- DOI: 10.1136/lupus-2023-000976
High-dimensional analysis of T-cell profiling variations following belimumab treatment in systemic lupus erythematosus
Abstract
Objective: This study sought to elucidate the molecular impacts of belimumab (BEL) treatment on T-cell immune profiling in SLE.
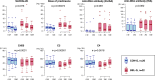
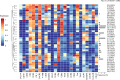
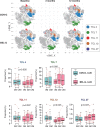
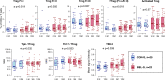
Methods: We used mass cytometry with 25 marker panels for T-cell immune profiling in peripheral blood T cells (CD3+) from 22 patients with BEL-treated SLE and 20 controls with non-BEL-treated SLE. An unsupervised machine-learning clustering, FlowSOM, was used to identify 39 T-cell clusters (TCLs; TCL01-TCL39). TCLs (% of CD3+) showing significant (p<0.05) associations with BEL treatment (BEL-TCL) were selected by a linear mixed-effects model for comparing groups of time-series data. Furthermore, we analysed the association between BEL treatment and variations in regulatory T-cell (Treg) phenotypes, and the ratio of other T-cell subsets to Treg as secondary analysis.
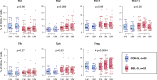
Results: Clinical outcomes: BEL treatment was associated with a decrease in daily prednisolone use (coef=-0.1769, p=0.00074), and an increase in serum CH50 (coef=0.4653, p=0.003), C3 (coef=1.1047, p=0.00001) and C4 (coef=0.2990, p=0.00157) levels. Molecular effects: five distinct BEL-TCLs (TCL 04, 07, 11, 12 and 27) were identified. Among these, BEL-treated patients exhibited increased proportions in the Treg-like cluster TCL11 (coef=0.404, p=0.037) and two naïve TCLs (TCL04 and TCL07). TCL27 showed increased levels (coef=0.222, p=0.037) inversely correlating with baseline C3 levels. Secondary analyses revealed associations between BEL treatment and an increase in Tregs (coef=1.749, p=0.0044), elevated proportions of the fraction of Tregs with inhibitory function (fTregs, coef=0.7294, p=0.0178) and changes in peripheral helper T cells/fTreg (coef=-4.475, p=0.0319) and T helper 17/fTreg ratios (coef=-6.7868, p=0.0327). Additionally, BEL was linked to variations in T-cell immunoglobulin and mucin domain-containing protein-3 expression (coef=0.2422, p=0.039).
Conclusions: The study suggests an association between BEL treatment and variations in T cells, particularly Tregs, in SLE pathologies involving various immune cells.
Keywords: T-lymphocytes, helper-inducer; autoimmunity; lupus erythematosus, systemic.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: AN reports personal fees from GlaxoSmithKline outside the submitted work. All authors declare that they have no known competing financial interests or personal relationships that could have potentially swayed the research presented in this paper.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
