Spinal-Specific Super Enhancer in Neuropathic Pain
- PMID: 37802656
- PMCID: PMC10711714
- DOI: 10.1523/JNEUROSCI.1006-23.2023
Spinal-Specific Super Enhancer in Neuropathic Pain
Abstract
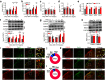
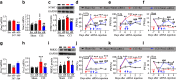
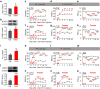
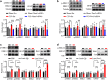
Dysfunctional gene expression in nociceptive pathways plays a critical role in the development and maintenance of neuropathic pain. Super enhancers (SEs), composed of a large cluster of transcriptional enhancers, are emerging as new players in the regulation of gene expression. However, whether SEs participate in nociceptive responses remains unknown. Here, we report a spinal-specific SE (SS-SE) that regulates chronic constriction injury (CCI)-induced neuropathic pain by driving Ntmt1 and Prrx2 transcription in dorsal horn neurons. Peripheral nerve injury significantly enhanced the activity of SS-SE and increased the expression of NTMT1 and PRRX2 in the dorsal horn of male mice in a bromodomain-containing protein 4 (BRD4)-dependent manner. Both intrathecal administration of a pharmacological BRD4 inhibitor JQ1 and CRISPR-Cas9-mediated SE deletion abolished the increased NTMT1 and PRRX2 in CCI mice and attenuated their nociceptive hypersensitivities. Furthermore, knocking down Ntmt1 or Prrx2 with siRNA suppressed the injury-induced elevation of phosphorylated extracellular-signal-regulated kinase (p-ERK) and glial fibrillary acidic protein (GFAP) expression in the dorsal horn and alleviated neuropathic pain behaviors. Mimicking the increase in spinal Ntmt1 or Prrx2 in naive mice increased p-ERK and GFAP expression and led to the genesis of neuropathic pain-like behavior. These results redefine our understanding of the regulation of pain-related genes and demonstrate that BRD4-driven increases in SS-SE activity is responsible for the genesis of neuropathic pain through the governance of NTMT1 and PRRX2 expression in dorsal horn neurons. Our findings highlight the therapeutic potential of BRD4 inhibitors for the treatment of neuropathic pain.SIGNIFICANCE STATEMENT SEs drive gene expression by recruiting master transcription factors, cofactors, and RNA polymerase, but their role in the development of neuropathic pain remains unknown. Here, we report that the activity of an SS-SE, located upstream of the genes Ntmt1 and Prrx2, was elevated in the dorsal horn of mice with neuropathic pain. SS-SE contributes to the genesis of neuropathic pain by driving expression of Ntmt1 and Prrx2 Both inhibition of SS-SE with a pharmacological BRD4 inhibitor and genetic deletion of SS-SE attenuated pain hypersensitivities. This study suggests an effective and novel therapeutic strategy for neuropathic pain.
Keywords: BRD4 inhibitor; Ntmt1; Prrx2; neuropathic pain; super enhancer.
Copyright © 2023 the authors.
Figures





References
-
- Achour M, Le Gras S, Keime C, Parmentier F, Lejeune F-X, Boutillier A-L, Néri C, Davidson I, Merienne K (2015) Neuronal identity genes regulated by super-enhancers are preferentially down-regulated in the striatum of Huntington's disease mice. Hum Mol Genet 24:3481–3496. 10.1093/hmg/ddv099 - DOI - PubMed
-
- Anand P, Brown JD, Lin CY, Qi J, Zhang R, Artero PC, Alaiti MA, Bullard J, Alazem K, Margulies KB, Cappola TP, Lemieux M, Plutzky J, Bradner JE, Haldar SM (2013) BET bromodomains mediate transcriptional pause release in heart failure. Cell 154:569–582. 10.1016/j.cell.2013.07.013 - DOI - PMC - PubMed
-
- Bai W-W, Tang Z-Y, Shan T-C, Jing X-J, Li P, Qin W-D, Song P, Wang B, Xu J, Liu Z, Yu H-Y, Ma Z-M, Wang S-X, Liu C, Guo T (2020) Up-regulation of paired-related homeobox 2 promotes cardiac fibrosis in mice following myocardial infarction by targeting of Wnt5a. J Cell Mol Med 24:2319–2329. 10.1111/jcmm.14914 - DOI - PMC - PubMed
-
- Bal E, Kumar R, Hadigol M, Holmes AB, Hilton LK, Loh JW, Dreval K, Wong JCH, Vlasevska S, Corinaldesi C, Soni RK, Basso K, Morin RD, Khiabanian H, Pasqualucci L, Dalla-Favera R (2022) Super-enhancer hypermutation alters oncogene expression in B cell lymphoma. Nature 607:808–815. 10.1038/s41586-022-04906-8 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
