Injectable and biodegradable piezoelectric hydrogel for osteoarthritis treatment
- PMID: 37802985
- PMCID: PMC10558537
- DOI: 10.1038/s41467-023-41594-y
Injectable and biodegradable piezoelectric hydrogel for osteoarthritis treatment
Abstract
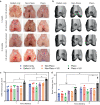
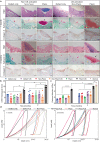
Osteoarthritis affects millions of people worldwide but current treatments using analgesics or anti-inflammatory drugs only alleviate symptoms of this disease. Here, we present an injectable, biodegradable piezoelectric hydrogel, made of short electrospun poly-L-lactic acid nanofibers embedded inside a collagen matrix, which can be injected into the joints and self-produce localized electrical cues under ultrasound activation to drive cartilage healing. In vitro, data shows that the piezoelectric hydrogel with ultrasound can enhance cell migration and induce stem cells to secrete TGF-β1, which promotes chondrogenesis. In vivo, the rabbits with osteochondral critical-size defects receiving the ultrasound-activated piezoelectric hydrogel show increased subchondral bone formation, improved hyaline-cartilage structure, and good mechanical properties, close to healthy native cartilage. This piezoelectric hydrogel is not only useful for cartilage healing but also potentially applicable to other tissue regeneration, offering a significant impact on the field of regenerative tissue engineering.
© 2023. Springer Nature Limited.
Conflict of interest statement
T.D.N. has a conflict of interest with PiezoBioMembrane Inc. and SingleTimeMicroneedles Inc. Eli Curry is currently an employee and common stock owner of Eli Lilly and Company. The work presented herein was completed prior to his employment with Eli Lilly and Company. Eli Curry is acting entirely on his own and any opinions or endeavors expressed herein are not in any manner affiliated with Eli Lilly and Company. T.V and T.D.N are inventors of the patent application PCT/US2021/053887. The remaining authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

