Amino acid transporter SLC38A5 is a tumor promoter and a novel therapeutic target for pancreatic cancer
- PMID: 37803043
- PMCID: PMC10558479
- DOI: 10.1038/s41598-023-43983-1
Amino acid transporter SLC38A5 is a tumor promoter and a novel therapeutic target for pancreatic cancer
Abstract
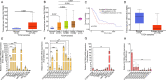
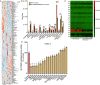
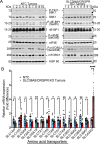
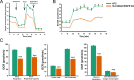
Pancreatic ductal adenocarcinoma (PDAC) cells have a great demand for nutrients in the form of sugars, amino acids, and lipids. Particularly, amino acids are critical for cancer growth and, as intermediates, connect glucose, lipid and nucleotide metabolism. PDAC cells meet these requirements by upregulating selective amino acid transporters. Here we show that SLC38A5 (SN2/SNAT5), a neutral amino acid transporter is highly upregulated and functional in PDAC cells. Using CRISPR/Cas9-mediated knockout of SLC38A5, we show its tumor promoting role in an in vitro cell line model as well as in a subcutaneous xenograft mouse model. Using metabolomics and RNA sequencing, we show significant reduction in many amino acid substrates of SLC38A5 as well as OXPHOS inactivation in response to SLC38A5 deletion. Experimental validation demonstrates inhibition of mTORC1, glycolysis and mitochondrial respiration in KO cells, suggesting a serious metabolic crisis associated with SLC38A5 deletion. Since many SLC38A5 substrates are activators of mTORC1 as well as TCA cycle intermediates/precursors, we speculate amino acid insufficiency as a possible link between SLC38A5 deletion and inactivation of mTORC1, glycolysis and mitochondrial respiration, and the underlying mechanism for PDAC attenuation. Overall, we show that SLC38A5 promotes PDAC, thereby identifying a novel, hitherto unknown, therapeutic target for PDAC.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
SLC38A5 Modulates Ferroptosis to Overcome Gemcitabine Resistance in Pancreatic Cancer.Cells. 2023 Oct 23;12(20):2509. doi: 10.3390/cells12202509. Cells. 2023. PMID: 37887353 Free PMC article.
-
Deletion of Slc6a14 reduces cancer growth and metastatic spread and improves survival in KPC mouse model of spontaneous pancreatic cancer.Biochem J. 2022 Mar 18;479(5):719-730. doi: 10.1042/BCJ20210855. Biochem J. 2022. PMID: 35212370 Free PMC article.
-
Amino acid transporter LAT1 in tumor-associated vascular endothelium promotes angiogenesis by regulating cell proliferation and VEGF-A-dependent mTORC1 activation.J Exp Clin Cancer Res. 2020 Nov 30;39(1):266. doi: 10.1186/s13046-020-01762-0. J Exp Clin Cancer Res. 2020. PMID: 33256804 Free PMC article.
-
The SLC38A5/SNAT5 amino acid transporter: from pathophysiology to pro-cancer roles in the tumor microenvironment.Am J Physiol Cell Physiol. 2023 Aug 1;325(2):C550-C562. doi: 10.1152/ajpcell.00169.2023. Epub 2023 Jul 17. Am J Physiol Cell Physiol. 2023. PMID: 37458433 Review.
-
SLC6A14 and SLC38A5 Drive the Glutaminolysis and Serine-Glycine-One-Carbon Pathways in Cancer.Pharmaceuticals (Basel). 2021 Mar 4;14(3):216. doi: 10.3390/ph14030216. Pharmaceuticals (Basel). 2021. PMID: 33806675 Free PMC article. Review.
Cited by
-
Induction of Oxidative Stress and Ferroptosis in Triple-Negative Breast Cancer Cells by Niclosamide via Blockade of the Function and Expression of SLC38A5 and SLC7A11.Antioxidants (Basel). 2024 Feb 27;13(3):291. doi: 10.3390/antiox13030291. Antioxidants (Basel). 2024. PMID: 38539825 Free PMC article.
-
Metabolic Signature of Warburg Effect in Cancer: An Effective and Obligatory Interplay between Nutrient Transporters and Catabolic/Anabolic Pathways to Promote Tumor Growth.Cancers (Basel). 2024 Jan 24;16(3):504. doi: 10.3390/cancers16030504. Cancers (Basel). 2024. PMID: 38339256 Free PMC article. Review.
-
SLC38A5 suppresses ferroptosis through glutamine-mediated activation of the PI3K/AKT/mTOR signaling in osteosarcoma.J Transl Med. 2024 Nov 7;22(1):1004. doi: 10.1186/s12967-024-05803-6. J Transl Med. 2024. PMID: 39511570 Free PMC article.
-
Impact of Oncogenic Changes in p53 and KRAS on Macropinocytosis and Ferroptosis in Colon Cancer Cells and Anticancer Efficacy of Niclosamide with Differential Effects on These Two Processes.Cells. 2024 May 30;13(11):951. doi: 10.3390/cells13110951. Cells. 2024. PMID: 38891084 Free PMC article.
-
Soybean Cyst Nematode-Resistant Protein AATRhg1 Affects Amino Acid Homeostasis and Betalain Accumulation.Plant Direct. 2025 Aug 26;9(8):e70098. doi: 10.1002/pld3.70098. eCollection 2025 Aug. Plant Direct. 2025. PMID: 40874116 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

