Long-Term Outcomes of Eladocagene Exuparvovec Compared with Standard of Care in Aromatic L-Amino Acid Decarboxylase (AADC) Deficiency: A Modelling Study
- PMID: 37803205
- PMCID: PMC10611606
- DOI: 10.1007/s12325-023-02689-6
Long-Term Outcomes of Eladocagene Exuparvovec Compared with Standard of Care in Aromatic L-Amino Acid Decarboxylase (AADC) Deficiency: A Modelling Study
Abstract
Introduction: Aromatic L-amino acid decarboxylase (AADC) deficiency is a rare disease with symptoms including movement disorders, developmental delays, and autonomic symptoms starting from birth; further, patients with AADC deficiency are at a high risk of death in the first decade of life. Limited information on the impact of treatment with gene therapy on patients' disease trajectories and survival, quality-of-life, and resource usage benefits are available.
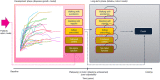
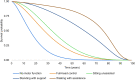
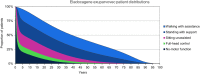
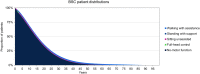
Method: A cohort-based model with a lifetime horizon has been developed, based on motor milestones, to estimate the long-term benefits for patients after treatment with eladocagene exuparvovec compared to best supportive care (BSC). The model takes a National Health Service (NHS) perspective using a UK setting. The model comprises two parts: the developmental phase, in which patients with initially no motor function can progress to other motor milestone states, and a long-term projection phase. Efficacy for eladocagene exuparvovec is derived from clinical trial data with a duration up to 120 months. As the incidence of AADC deficiency is low, data for key model inputs is lacking; therefore estimates of survival by motor milestone were based on proxy diseases. A disease-specific utility study provided quality of life inputs and a burden of illness study informed inputs for disease management.
Results: The model indicates survival (25.25 undiscounted life years gained) and quality-of-life benefits (20.21 undiscounted quality-adjusted life years [QALYs] gained) for patients treated with eladocagene exuparvovec compared to BSC. Resource usage costs are greater for patients treated with eladocagene exuparvovec, mainly due to the increased life expectancy during which patients accrue additional healthcare resource usage. Scenario analyses indicate robust results.
Conclusion: This study assessed long-term outcomes for patients with AADC deficiency. Patients treated with eladocagene exuparvovec were found to have improved survival and quality of life benefits compared to patients treated with BSC.
Keywords: AADC deficiency; Aromatic L-amino acid decarboxylase deficiency; Eladocagene exuparvovec; Long-term modelling; Markov modelling; Quality of life; Survival.
© 2023. The Author(s).
Conflict of interest statement
Authors Claire L Simons, Martijn JHG Simons, and Craig Bennison acted as consultants to PTC Therapeutics through their employment with OPEN Health Group and declare they have no personal, commercial, academic, or financial conflicts of interest. Rongrong Zhang and Mats Bergkvist are employees of PTC Therapeutics. Wuh-Liang Hwu participated as an advisory board member, received consulting fees, and was a speaker for PTC Therapeutics, BioMarin, and Sanofi. He was a grant recipient for PTC Therapeutics and BioMarin and a research investigator for PTC Therapeutics.
Figures


References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

