High-flow nasal cannula (HFNC) vs continuous positive airway pressure (CPAP) vs nasal intermittent positive pressure ventilation as primary respiratory support in infants of ≥ 32 weeks gestational age (GA): study protocol for a three-arm multi-center randomized controlled trial
- PMID: 37803402
- PMCID: PMC10557210
- DOI: 10.1186/s13063-023-07665-7
High-flow nasal cannula (HFNC) vs continuous positive airway pressure (CPAP) vs nasal intermittent positive pressure ventilation as primary respiratory support in infants of ≥ 32 weeks gestational age (GA): study protocol for a three-arm multi-center randomized controlled trial
Abstract
Background: Health problems in neonates with gestational age (GA) ≥ 32 weeks remain a major medical concern. Respiratory distress (RD) is one of the common reasons for admission of neonates with GA ≥ 32 weeks. Noninvasive ventilation (NIV) represents a crucial approach to treat RD, and currently, the most used NIV modes in neonatal intensive care unit include high-flow nasal cannula (HFNC), continuous positive airway pressure (CPAP), and nasal intermittent positive pressure ventilation. Although extensive evidence supports the use of NIPPV in neonates with a GA < 32 weeks, limited data exist regarding its effectiveness in neonates with GA ≥ 32 weeks. Therefore, the aim of this study is to compare the clinical efficacy of HFNC, CPAP, and NIPPV as primary NIV in neonates with GA ≥ 32 weeks who experience RD.
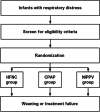
Methods: This trial is designed as an assessor-blinded, three-arm, multi-center, parallel, randomized controlled trial, conducted in neonates ≥ 32 weeks' GA requiring primary NIV in the first 24 h of life. The neonates will be randomly assigned to one of three groups: HFNC, CPAP or NIPPV group. The effectiveness, safety and comfort of NIV will be evaluated. The primary outcome is the occurrence of treatment failure within 72 h after enrollment. Secondary outcomes include death before discharge, surfactant treatment within 72 h after randomization, duration of both noninvasive and invasive mechanical ventilation, duration of oxygen therapy, bronchopulmonary dysplasia, time to achieve full enteral nutrition, necrotizing enterocolitis, duration of admission, cost of admission, air leak syndrome, nasal trauma, and comfort score.
Discussion: Currently, there is a paucity of data regarding the utilization of NIPPV in neonates with GA ≥ 32 weeks. This study will provide clinical evidence for the development of respiratory treatment strategies in neonates at GA ≥ 32 weeks with RD, with the aim of minimizing the incidence of tracheal intubation and reducing the complications associated with NIV.
Trial registration: Chinese Clinical Trial Registry: ChiCTR2300069192. Registered on March 9, 2023, https://www.chictr.org.cn/showproj.html?proj=171491 .
Keywords: Continuous positive airway pressure; High-flow nasal cannula; Nasal intermittent positive pressure ventilation; Neonates; Noninvasive ventilation; Randomised controlled trial.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller A-B, Narwal R, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379(9832):2162–2172. - PubMed
-
- Ersch J, Roth-Kleiner M, Baeckert P, Bucher HU. Increasing incidence of respiratory distress in neonates. Acta Paediatr. 2007;96(11):1577–1581. - PubMed
-
- Bricelj K, Tul N, Lasic M, Bregar AT, Verdenik I, Lucovnik M, et al. Respiratory morbidity in twins by birth order, gestational age and mode of delivery. J Perinat Med. 2016;44(8):899–902. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

