Outcomes of patients with active cancers and pre-existing cardiovascular diseases infected with SARS-CoV-2
- PMID: 37803479
- PMCID: PMC10557272
- DOI: 10.1186/s40959-023-00187-w
Outcomes of patients with active cancers and pre-existing cardiovascular diseases infected with SARS-CoV-2
Abstract
Objective: To determine the impact of acute SARS-CoV-2 infection on patient with concomitant active cancer and CVD.
Methods: The researchers extracted and analyzed data from the National COVID Cohort Collaborative (N3C) database between January 1, 2020, and July 22, 2022. They included only patients with acute SARS-CoV-2 infection, defined as a positive test by PCR 21 days before and 5 days after the day of index hospitalization. Active cancers were defined as last cancer drug administered within 30 days of index admission. The "Cardioonc" group consisted of patients with CVD and active cancers. The cohort was divided into four groups: (1) CVD (-), (2) CVD ( +), (3) Cardioonc (-), and (4) Cardioonc ( +), where (-) or ( +) denotes acute SARS-CoV-2 infection status. The primary outcome of the study was major adverse cardiovascular events (MACE), including acute stroke, acute heart failure, myocardial infarction, or all-cause mortality. The researchers analyzed the outcomes by different phases of the pandemic and performed competing-risk analysis for other MACE components and death as a competing event.
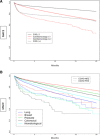
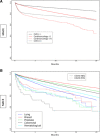
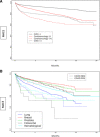
Results: The study analyzed 418,306 patients, of which 74%, 10%, 15.7%, and 0.3% had CVD (-), CVD ( +), Cardioonc (-), and Cardioonc ( +), respectively. The Cardioonc ( +) group had the highest MACE events in all four phases of the pandemic. Compared to CVD (-), the Cardioonc ( +) group had an odds ratio of 1.66 for MACE. However, during the Omicron era, there was a statistically significant increased risk for MACE in the Cardioonc ( +) group compared to CVD (-). Competing risk analysis showed that all-cause mortality was significantly higher in the Cardioonc ( +) group and limited other MACE events from occurring. When the researchers identified specific cancer types, patients with colon cancer had higher MACE.
Conclusion: In conclusion, the study found that patients with both CVD and active cancer suffered relatively worse outcomes when they had acute SARS-CoV-2 infection during early and alpha surges in the United States. These findings highlight the need for improved management strategies and further research to better understand the impact of the virus on vulnerable populations during the COVID-19 pandemic.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
The authors declare no competing interests.
Figures


Update of
-
Outcomes of Patients with Active Cancers and Pre-Existing Cardiovascular Diseases Infected with SARS-CoV-2.Res Sq [Preprint]. 2023 May 23:rs.3.rs-2952641. doi: 10.21203/rs.3.rs-2952641/v1. Res Sq. 2023. Update in: Cardiooncology. 2023 Oct 6;9(1):36. doi: 10.1186/s40959-023-00187-w. PMID: 37292998 Free PMC article. Updated. Preprint.
References
-
- Ganatra S, Dani SS, Redd R, et al. Outcomes of COVID-19 in patients with a history of cancer and comorbid cardiovascular disease. J Natl Compr Canc Netw. 2020;19(4):1–10. - PubMed
Grants and funding
- UL1 TR001445/TR/NCATS NIH HHS/United States
- UL1 TR001412/TR/NCATS NIH HHS/United States
- UL1 TR001442/TR/NCATS NIH HHS/United States
- UL1 TR001866/TR/NCATS NIH HHS/United States
- UL1 TR003167/TR/NCATS NIH HHS/United States
- UL1 TR001449/TR/NCATS NIH HHS/United States
- UL1 TR001433/TR/NCATS NIH HHS/United States
- UL1 TR001860/TR/NCATS NIH HHS/United States
- U54 GM104942/GM/NIGMS NIH HHS/United States
- UL1 TR002366/TR/NCATS NIH HHS/United States
- U54 GM115677/GM/NIGMS NIH HHS/United States
- UL1 TR001872/TR/NCATS NIH HHS/United States
- UL1 TR001878/TR/NCATS NIH HHS/United States
- UL1 TR002529/TR/NCATS NIH HHS/United States
- UL1 TR001863/TR/NCATS NIH HHS/United States
- U54 GM115516/GM/NIGMS NIH HHS/United States
- UL1 TR002541/TR/NCATS NIH HHS/United States
- U54 GM104940/GM/NIGMS NIH HHS/United States
- UL1 TR003107/TR/NCATS NIH HHS/United States
- 5U54GM104942-04/GM/NIGMS NIH HHS/United States
- U24 TR002306/TR/NCATS NIH HHS/United States
- UL1 TR001876/TR/NCATS NIH HHS/United States
- UL1 TR001436/TR/NCATS NIH HHS/United States
- UL1 TR002378/TR/NCATS NIH HHS/United States
- UL1 TR002384/TR/NCATS NIH HHS/United States
- UL1 TR001414/TR/NCATS NIH HHS/United States
- UL1 TR002389/TR/NCATS NIH HHS/United States
- UL1 TR002550/TR/NCATS NIH HHS/United States
- UL1 TR001425/TR/NCATS NIH HHS/United States
- UL1 TR002373/TR/NCATS NIH HHS/United States
- UL1 TR002556/TR/NCATS NIH HHS/United States
- UL1 TR003017/TR/NCATS NIH HHS/United States
- UL1 TR001881/TR/NCATS NIH HHS/United States
- UL1 TR002645/TR/NCATS NIH HHS/United States
- UL1 TR001430/TR/NCATS NIH HHS/United States
- UL1 TR003142/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous
