EAAT3 impedes oligodendrocyte remyelination in chronic cerebral hypoperfusion-induced white matter injury
- PMID: 37803915
- PMCID: PMC10805396
- DOI: 10.1111/cns.14487
EAAT3 impedes oligodendrocyte remyelination in chronic cerebral hypoperfusion-induced white matter injury
Abstract
Background: Chronic cerebral hypoperfusion-induced demyelination causes progressive white matter injury, although the pathogenic pathways are unknown.
Methods: The Single Cell Portal and PanglaoDB databases were used to analyze single-cell RNA sequencing experiments to determine the pattern of EAAT3 expression in CNS cells. Immunofluorescence (IF) was used to detect EAAT3 expression in oligodendrocytes and oligodendrocyte progenitor cells (OPCs). EAAT3 levels in mouse brains were measured using a western blot at various phases of development, as well as in traumatic brain injury (TBI) and intracerebral hemorrhage (ICH) mouse models. The mouse bilateral carotid artery stenosis (BCAS) model was used to create white matter injury. IF, Luxol Fast Blue staining, and electron microscopy were used to investigate the effect of remyelination. 5-Ethynyl-2-Deoxy Uridine staining, transwell chamber assays, and IF were used to examine the effects of OPCs' proliferation, migration, and differentiation in vivo and in vitro. The novel object recognition test, the Y-maze test, the rotarod test, and the grid walking test were used to examine the impact of behavioral modifications.
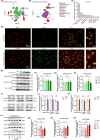
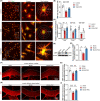
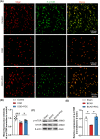
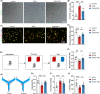
Results: A considerable amount of EAAT3 was expressed in OPCs and mature oligodendrocytes, according to single-cell RNA sequencing data. During multiple critical phases of mouse brain development, there were no substantial changes in EAAT3 levels in the hippocampus, cerebral cortex, or white matter. Furthermore, neither the TBI nor ICH models significantly affected the levels of EAAT3 in the aforementioned brain areas. The chronic white matter injury caused by BCAS, on the other hand, resulted in a strikingly high level of EAAT3 expression in the oligodendroglia and white matter. Correspondingly, blocking EAAT3 assisted in the recovery of cognitive and motor impairment as well as the restoration of cerebral blood flow following BCAS. Furthermore, EAAT3 suppression was connected to improved OPCs' survival and proliferation in vivo as well as faster OPCs' proliferation, migration, and differentiation in vitro. Furthermore, this study revealed that the mTOR pathway is implicated in EAAT3-mediated remyelination.
Conclusions: Our findings provide the first evidence that abnormally high levels of oligodendroglial EAAT3 in chronic cerebral hypoperfusion impair OPCs' pro-remyelination actions, hence impeding white matter repair and functional recovery. EAAT3 inhibitors could be useful in the treatment of ischemia demyelination.
Keywords: chronic cerebral hypoperfusion; excitatory amino acid transporter 3 (EAAT3); neuroprotection; oligodendrocyte progenitor cells (OPCs); white matter.
© 2023 The Authors. CNS Neuroscience & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this study.
Figures




References
-
- Dong Y, Li Y, Liu K, et al. Anosmia, mild cognitive impairment, and biomarkers of brain aging in older adults. Alzheimers Dement. 2023;19(2):589‐601. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- Guangxi Research and Innovation Base for Basic and Clinical Application of Nerve Injury and Repair Project
- Guangxi Science and Technology Project
- National Natural Science Foundation of China
- The Basic Ability Enhancement Program for Young and Middle-aged Teachers of Guangxi
- the Plan Project of Guilin Scientific Research and Technology Development
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

