Focused ultrasound as an alternative to dry needling for the treatment of tendinopathies: A murine model
- PMID: 37804211
- PMCID: PMC10932869
- DOI: 10.1002/jor.25700
Focused ultrasound as an alternative to dry needling for the treatment of tendinopathies: A murine model
Abstract
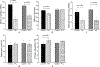
Tendinopathies account for 30% of 102 million annual musculoskeletal injuries occurring annually in the United States. Current treatments, like dry needling, induce microdamage to promote healing but produce mixed success rates. Previously, we showed focused ultrasound can noninvasively create microdamage while preserving mechanical properties in ex vivo murine tendons. This present study compared growth factor, histological, and mechanical effects after focused ultrasound or dry needling treatments in an in vivo murine tendon injury model. Partial Achilles tenotomy was performed in 26 rats. One-week postsurgery, tendons were treated with focused ultrasound (1.5 MHz, 1-ms pulses at 10 Hz for 106 s, p+ = 49 MPa, p- = 19 MPa) or dry needling (30 G needle, 5 fenestrations over 20 s) and survived for 1 additional week. Blood was collected immediately before and after treatment and before euthanasia; plasma was assayed for growth factors. Treated tendons and contralateral controls were harvested for histology or mechanical testing. No differences were found between treatments in release of insulin growth factor 1 and transforming growth factor beta; vascular endothelial growth factor A concentrations were too low for detection. Histologically, focused ultrasound and dry needling tendons displayed localized fibroblast infiltration without collagen proliferation with no detectable differences between treatments. Mechanically, stiffness and percent relaxation of dry needling tendons were lower than controls (p = 0.0041, p = 0.0441, respectively), whereas stiffness and percent relaxation of focused ultrasound tendons were not different from controls. These results suggest focused ultrasound should be studied further to determine how this modality can be leveraged as a therapy for tendinopathies.
Keywords: dry needling (DN); focused ultrasound (fUS); growth factors; histology; mechanical testing.
© 2023 The Authors. Journal of Orthopaedic Research® published by Wiley Periodicals LLC on behalf of Orthopaedic Research Society.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures

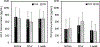


Similar articles
-
Effects of focused ultrasound and dry needling on tendon mechanical properties.J Biomech. 2022 Feb;132:110934. doi: 10.1016/j.jbiomech.2021.110934. Epub 2021 Dec 22. J Biomech. 2022. PMID: 34995989 Free PMC article.
-
Comparison between dry needling and focused ultrasound on the mechanical properties of the rat Achilles tendon: A pilot study.J Biomech. 2021 May 7;120:110384. doi: 10.1016/j.jbiomech.2021.110384. Epub 2021 Mar 15. J Biomech. 2021. PMID: 33773298 Free PMC article.
-
Focused Ultrasound Mechanical Disruption of Ex Vivo Rat Tendon.IEEE Trans Ultrason Ferroelectr Freq Control. 2021 Sep;68(9):2981-2986. doi: 10.1109/TUFFC.2021.3075375. Epub 2021 Aug 27. IEEE Trans Ultrason Ferroelectr Freq Control. 2021. PMID: 33891552 Free PMC article.
-
Dry Needling as a Treatment Modality for Tendinopathy: a Narrative Review.Curr Rev Musculoskelet Med. 2020 Feb;13(1):133-140. doi: 10.1007/s12178-020-09608-0. Curr Rev Musculoskelet Med. 2020. PMID: 31942676 Free PMC article. Review.
-
Therapeutic Ultrasound and Shockwave Therapy for Tendinopathy: A Narrative Review.Am J Phys Med Rehabil. 2022 Aug 1;101(8):801-807. doi: 10.1097/PHM.0000000000001894. Epub 2021 Oct 4. Am J Phys Med Rehabil. 2022. PMID: 35859290 Free PMC article. Review.
References
-
- Yelin E, Weinstein S, King T. 2016. The burden of musculoskeletal diseases in the United States. Semin. Arthritis Rheum. 46(3):259–260. - PubMed
-
- Komi PV, Fukashiro S, Jarvinen M. 1992. Biomechanical Loading of Achilles Tendon During Normal Locomotion. Clin. Sports Med. 11(3):521–531. - PubMed
-
- Sharma P, Maffulli N. 2005. Tendon injury and tendinopathy: healing and repair. J Bone Joint Surg. 87(1):187–202. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

