Cortical origin of theta error signals
- PMID: 37804250
- PMCID: PMC10690871
- DOI: 10.1093/cercor/bhad367
Cortical origin of theta error signals
Abstract
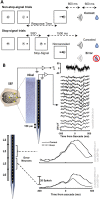
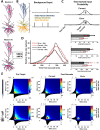
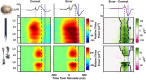
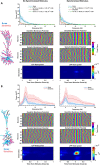
A multi-scale approach elucidated the origin of the error-related-negativity (ERN), with its associated theta-rhythm, and the post-error-positivity (Pe) in macaque supplementary eye field (SEF). Using biophysical modeling, synaptic inputs to a subpopulation of layer-3 (L3) and layer-5 (L5) pyramidal cells (PCs) were optimized to reproduce error-related spiking modulation and inter-spike intervals. The intrinsic dynamics of dendrites in L5 but not L3 error PCs generate theta rhythmicity with random phases. Saccades synchronized the phases of the theta-rhythm, which was magnified on errors. Contributions from error PCs to the laminar current source density (CSD) observed in SEF were negligible and could not explain the observed association between error-related spiking modulation in L3 PCs and scalp-EEG. CSD from recorded laminar field potentials in SEF was comprised of multipolar components, with monopoles indicating strong electro-diffusion, dendritic/axonal electrotonic current leakage outside SEF, or violations of the model assumptions. Our results also demonstrate the involvement of secondary cortical regions, in addition to SEF, particularly for the later Pe component. The dipolar component from the observed CSD paralleled the ERN dynamics, while the quadrupolar component paralleled the Pe. These results provide the most advanced explanation to date of the cellular mechanisms generating the ERN.
Keywords: CSD; ERN; biophysical models; multiscale analysis; theta rhythm.
© The Author(s) 2023. Published by Oxford University Press. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures



References
-
- Armstrong IT, Munoz DP. Inhibitory control of eye movements during oculomotor countermanding in adults with attention-deficit hyperactivity disorder. Exp Brain Res. 2003:152(4):444–452. - PubMed
-
- Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat Neurosci. 2003:6(2):115–116. - PubMed
-
- Billeh YN, Cai B, Gratiy SL, Dai K, Iyer R, Gouwens NW, Abbasi-Asl R, Jia X, Siegle JH, Olsen SR, et al. Systematic integration of structural and functional data into multi-scale models of mouse primary visual cortex. Neuron. 2020:106:388–403.e18. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

