Sustained OMA1-mediated integrated stress response is beneficial for spastic ataxia type 5
- PMID: 37804316
- PMCID: PMC10907083
- DOI: 10.1093/brain/awad340
Sustained OMA1-mediated integrated stress response is beneficial for spastic ataxia type 5
Abstract
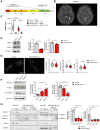
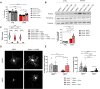
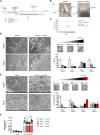
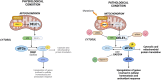
AFG3L2 is a mitochondrial protease exerting protein quality control in the inner mitochondrial membrane. Heterozygous AFG3L2 mutations cause spinocerebellar ataxia type 28 (SCA28) or dominant optic atrophy type 12 (DOA12), while biallelic AFG3L2 mutations result in the rare and severe spastic ataxia type 5 (SPAX5). The clinical spectrum of SPAX5 includes childhood-onset cerebellar ataxia, spasticity, dystonia and myoclonic epilepsy. We previously reported that the absence or mutation of AFG3L2 leads to the accumulation of mitochondria-encoded proteins, causing the overactivation of the stress-sensitive protease OMA1, which over-processes OPA1, leading to mitochondrial fragmentation. Recently, OMA1 has been identified as the pivotal player communicating mitochondrial stress to the cytosol via a pathway involving the inner mitochondrial membrane protein DELE1 and the cytosolic kinase HRI, thus eliciting the integrated stress response. In general, the integrated stress response reduces global protein synthesis and drives the expression of cytoprotective genes that allow cells to endure proteotoxic stress. However, the relevance of the OMA1-DELE1-HRI axis in vivo, and especially in a human CNS disease context, has been poorly documented thus far. In this work, we demonstrated that mitochondrial proteotoxicity in the absence/mutation of AFG3L2 activates the OMA1-DELE1-HRI pathway eliciting the integrated stress response. We found enhanced OMA1-dependent processing of DELE1 upon depletion of AFG3L2. Also, in both skin fibroblasts from SPAX5 patients (including a novel case) and in the cerebellum of Afg3l2-/- mice we detected increased phosphorylation of the α-subunit of the eukaryotic translation initiation factor 2 (eIF2α), increased levels of ATF4 and strong upregulation of its downstream targets (Chop, Chac1, Ppp1r15a and Ffg21). Silencing of DELE1 or HRI in SPAX5 fibroblasts (where OMA1 is overactivated at basal state) reduces eIF2α phosphorylation and affects cell growth. In agreement, pharmacological potentiation of integrated stress response via Sephin-1, a drug that selectively inhibits the stress-induced eIF2alpha phosphatase GADD34 (encoded by Ppp1r15a), improved cell growth of SPAX5 fibroblasts and cell survival and dendritic arborization ex vivo in primary Afg3l2-/- Purkinje neurons. Notably, Sephin-1 treatment in vivo extended the lifespan of Afg3l2-/- mice, improved Purkinje neuron morphology, mitochondrial ultrastructure and respiratory capacity. These data indicate that activation of the OMA1-DELE1-HRI pathway is protective in the context of SPAX5. Pharmacological tuning of the integrated stress response may represent a future therapeutic strategy for SPAX5 and other cerebellar ataxias caused by impaired mitochondrial proteostasis.
Keywords: OMA1; integrated stress response; spastic ataxia type 5.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
The authors report no competing interests.
Figures


References
-
- Quiros PM, Langer T, Lopez-Otin C. New roles for mitochondrial proteases in health, ageing and disease. Nat Rev Mol Cell Biol. 2015;16:345–359. - PubMed
-
- Brussino A, Brusco A, Durr A, et al. Spinocerebellar ataxia type 28. In: Pagon RA, Adam MP, Ardinger HH, eds. GeneReviews®. University of Washington, Seattle; 1993. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

