Vertebrate-class-specific binding modes of the alphavirus receptor MXRA8
- PMID: 37804831
- PMCID: PMC10615782
- DOI: 10.1016/j.cell.2023.09.007
Vertebrate-class-specific binding modes of the alphavirus receptor MXRA8
Abstract
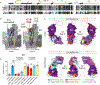
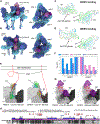
MXRA8 is a receptor for chikungunya (CHIKV) and other arthritogenic alphaviruses with mammalian hosts. However, mammalian MXRA8 does not bind to alphaviruses that infect humans and have avian reservoirs. Here, we show that avian, but not mammalian, MXRA8 can act as a receptor for Sindbis, western equine encephalitis (WEEV), and related alphaviruses with avian reservoirs. Structural analysis of duck MXRA8 complexed with WEEV reveals an inverted binding mode compared with mammalian MXRA8 bound to CHIKV. Whereas both domains of mammalian MXRA8 bind CHIKV E1 and E2, only domain 1 of avian MXRA8 engages WEEV E1, and no appreciable contacts are made with WEEV E2. Using these results, we generated a chimeric avian-mammalian MXRA8 decoy-receptor that neutralizes infection of multiple alphaviruses from distinct antigenic groups in vitro and in vivo. Thus, different alphaviruses can bind MXRA8 encoded by different vertebrate classes with distinct engagement modes, which enables development of broad-spectrum inhibitors.
Keywords: alphavirus; birds; cryoelectron microscopy; evolution; inhibitor; mammals; pathogenesis; receptor; species; structure; tropism.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests M.S.D. is a consultant to or member of a Scientific Advisory Board for Inbios, Ocugen, Vir Biotechnology, Topspin Therapeutics, and Moderna. The Diamond laboratory has received unrelated funding support in sponsored research agreements from Emergent BioSolutions, Moderna, Generate Biomedicines, Vir Biotechnology, and Immunome. D.H.F. is a founder of Courier Therapeutics and his laboratory has received unrelated funding support from Emergent BioSolutions and Mallinckrodt Pharmaceuticals. J.M.E. is an employee of Vir Biotechnology.
Figures

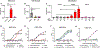



References
-
- Weaver SCS, D.W. (2011). Alphavirus Infections. In Tropical Infectious Diseases: Principles, Pathogens and Practice, D.H.W. Guerrant Richard L., Weller Peter F., ed. (Saunders WB), pp. 519–524. 10.1016/B978-0-7020-3935-5.00078-1. - DOI
-
- Strauss JHSEG (1997). Recombination in Alphaviruses. Seminars in VIROLOGY 8,, 85–94, VI9701151044.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

