Generative replay underlies compositional inference in the hippocampal-prefrontal circuit
- PMID: 37804832
- PMCID: PMC10914680
- DOI: 10.1016/j.cell.2023.09.004
Generative replay underlies compositional inference in the hippocampal-prefrontal circuit
Abstract
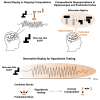
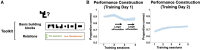
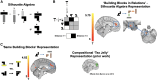
Human reasoning depends on reusing pieces of information by putting them together in new ways. However, very little is known about how compositional computation is implemented in the brain. Here, we ask participants to solve a series of problems that each require constructing a whole from a set of elements. With fMRI, we find that representations of novel constructed objects in the frontal cortex and hippocampus are relational and compositional. With MEG, we find that replay assembles elements into compounds, with each replay sequence constituting a hypothesis about a possible configuration of elements. The content of sequences evolves as participants solve each puzzle, progressing from predictable to uncertain elements and gradually converging on the correct configuration. Together, these results suggest a computational bridge between apparently distinct functions of hippocampal-prefrontal circuitry and a role for generative replay in compositional inference and hypothesis testing.
Keywords: cognitive maps; compositional inference; flexible reasoning; neural replay; prefrontal-hippocampal circuit.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests Z.K.-N. and M.B. are employed by DeepMind Technologies Limited.
Figures







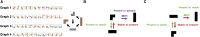

References
-
- Stachenfeld K.L., Botvinick M.M., Gershman S.J. The hippocampus as a predictive map. Nat. Neurosci. 2017;20:1643–1653. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

