Deep-brain optical recording of neural dynamics during behavior
- PMID: 37804833
- PMCID: PMC10843303
- DOI: 10.1016/j.neuron.2023.09.006
Deep-brain optical recording of neural dynamics during behavior
Abstract
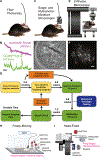
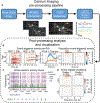
In vivo fluorescence recording techniques have produced landmark discoveries in neuroscience, providing insight into how single cell and circuit-level computations mediate sensory processing and generate complex behaviors. While much attention has been given to recording from cortical brain regions, deep-brain fluorescence recording is more complex because it requires additional measures to gain optical access to harder to reach brain nuclei. Here we discuss detailed considerations and tradeoffs regarding deep-brain fluorescence recording techniques and provide a comprehensive guide for all major steps involved, from project planning to data analysis. The goal is to impart guidance for new and experienced investigators seeking to use in vivo deep fluorescence optical recordings in awake, behaving rodent models.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests M.R.B. is a co-founder and scientific advisory board member of Neurolux, Inc, a Neurotechnology company. None of the research noted here is related to those efforts.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

