Blood-based tests for multicancer early detection (PATHFINDER): a prospective cohort study
- PMID: 37805216
- PMCID: PMC11027492
- DOI: 10.1016/S0140-6736(23)01700-2
Blood-based tests for multicancer early detection (PATHFINDER): a prospective cohort study
Abstract
Background: Multicancer early detection (MCED) blood tests can detect a cancer signal from circulating cell-free DNA (cfDNA). PATHFINDER was a prospective cohort study investigating the feasibility of MCED testing for cancer screening.
Methods: In this prospective cohort study done in oncology and primary care outpatient clinics at seven US health networks, a convenience sample of adults aged 50 years or older without signs or symptoms of cancer consented to MCED testing. We collected blood, analysed cfDNA, and returned results to participants' doctors. If a methylation signature indicative of cancer was detected, predicted cancer signal origin(s) informed diagnostic assessment. The primary outcome was time to, and extent of, diagnostic testing required to confirm the presence or absence of cancer. This trial is registered at ClinicalTrials.gov, NCT04241796, and is completed.
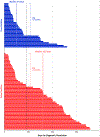
Findings: Between Dec 12, 2019, and Dec 4, 2020, we recruited 6662 participants. 4204 (63·5%) of 6621 participants with analysable results were women, 2417 (36·5%) were men, and 6071 (91·7%) were White. A cancer signal was detected in 92 (1·4%) of 6621 participants with analysable results. 35 (38%) participants were diagnosed with cancer (true positives) and 57 (62%) had no cancer diagnosis (false positives). Excluding two participants whose diagnostic assessments began before MCED test results were reported, median time to diagnostic resolution was 79 days (IQR 37-219): 57 days (33-143) in true-positive and 162 days (44-248) in false-positive participants. Most participants had both laboratory tests (26 [79%] of 33 with true-positive results and 50 [88%] of 57 with false-positive results) and imaging (30 [91%] of 33 with true-positive results and 53 [93%] of 57 with false-positive results). Fewer procedures were done in participants with false-positive results (17 [30%] of 57) than true-positive results (27 [82%] of 33) and few had surgery (one with a false-positive result and three with a true-positive result).
Interpretation: This study supports the feasibility of MCED screening for cancer and underscores the need for further research investigating the test's clinical utility.
Funding: GRAIL.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests DS reports an uncompensated advisory role and serving as a study investigator with research funding to Dana Farber Cancer Institute (GRAIL) and personal fees for editorial service (Journal of the American Medical Association). TMB reports a consultant agreement with GRAIL, AbbVie, Amgen, Arvinas, Astellas Pharma, AstraZeneca, Bayer, Bristol Myers Squib, Constellation, Dantari Pharmaceuticals, GlaxoSmithKline, Janssen, Myovant Sciences, Pfizer, Sanofi, and Sapience Therapeutics; and reports stock ownership in Arvinas, Salarius Pharmaceuticals, and Exact Sciences. CHM reports a consultant agreement with GRAIL. LN reports stock ownership in Culmination Bio. KCC reports stock ownership in Illumina, Bristol Myers Squib, Gilead, Baxter, and Bayer, and is an employee of GRAIL. ETF and ML report stock ownership of Illumina and are employees of GRAIL. EAK is an employee of GRAIL. All other authors declare no competing interests.
Figures


Comment in
-
PATHFINDER: another step on the uncharted path to multicancer screening.Lancet. 2023 Oct 7;402(10409):1213-1215. doi: 10.1016/S0140-6736(23)02050-0. Lancet. 2023. PMID: 37805199 No abstract available.
References
-
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin 2023;73(1):17–48. - PubMed
-
- Clarke CA, Hubbell E, Kurian AW, Colditz GA, Hartman AR, Gomez SL. Projected Reductions in Absolute Cancer-Related Deaths from Diagnosing Cancers Before Metastasis, 2006–2015. Cancer Epidemiol Biomarkers Prev 2020;29(5):895–902. - PubMed
-
- Beer TM. Novel blood-based early cancer detection: diagnostics in development. Am J Manag Care 2020;26(14 Suppl):S292–S9. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

