mRNA-based vaccines and therapeutics: an in-depth survey of current and upcoming clinical applications
- PMID: 37805495
- PMCID: PMC10559634
- DOI: 10.1186/s12929-023-00977-5
mRNA-based vaccines and therapeutics: an in-depth survey of current and upcoming clinical applications
Abstract
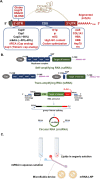
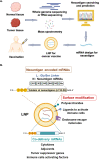
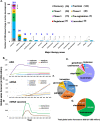
mRNA-based drugs have tremendous potential as clinical treatments, however, a major challenge in realizing this drug class will promise to develop methods for safely delivering the bioactive agents with high efficiency and without activating the immune system. With regard to mRNA vaccines, researchers have modified the mRNA structure to enhance its stability and promote systemic tolerance of antigenic presentation in non-inflammatory contexts. Still, delivery of naked modified mRNAs is inefficient and results in low levels of antigen protein production. As such, lipid nanoparticles have been utilized to improve delivery and protect the mRNA cargo from extracellular degradation. This advance was a major milestone in the development of mRNA vaccines and dispelled skepticism about the potential of this technology to yield clinically approved medicines. Following the resounding success of mRNA vaccines for COVID-19, many other mRNA-based drugs have been proposed for the treatment of a variety of diseases. This review begins with a discussion of mRNA modifications and delivery vehicles, as well as the factors that influence administration routes. Then, we summarize the potential applications of mRNA-based drugs and discuss further key points pertaining to preclinical and clinical development of mRNA drugs targeting a wide range of diseases. Finally, we discuss the latest market trends and future applications of mRNA-based drugs.
Keywords: Administration routes; Lipid nanoparticles; Targeting mRNA delivery system; mRNA therapeutics; mRNA vaccine.
© 2023. National Science Council of the Republic of China (Taiwan).
Conflict of interest statement
The authors have declared no competing interests.
Figures



References
-
- Arance Fernandez ANAM, Vulsteke JF, Rutten C, Soria A, Carrasco A, Neyns J, Keersmaecker B, Assche BD, Lindmark B. A phase I study (E011-Mel) of a trimix-based mRNA immunotherapy (Eci-006) in resected melanoma patients: analysis of safety and immunogenicity. J Clin Oncol. 2019;37:S2645. doi: 10.1200/JCO.2019.37.15_suppl.2641. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

