Metabolic phenotyping of BMI to characterize cardiometabolic risk: evidence from large population-based cohorts
- PMID: 37805498
- PMCID: PMC10560260
- DOI: 10.1038/s41467-023-41963-7
Metabolic phenotyping of BMI to characterize cardiometabolic risk: evidence from large population-based cohorts
Abstract
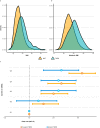
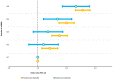
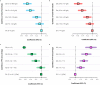
Obesity is a risk factor for type 2 diabetes and cardiovascular disease. However, a substantial proportion of patients with these conditions have a seemingly normal body mass index (BMI). Conversely, not all obese individuals present with metabolic disorders giving rise to the concept of "metabolically healthy obese". We use lipidomic-based models for BMI to calculate a metabolic BMI score (mBMI) as a measure of metabolic dysregulation associated with obesity. Using the difference between mBMI and BMI (mBMIΔ), we identify individuals with a similar BMI but differing in their metabolic health and disease risk profiles. Exercise and diet associate with mBMIΔ suggesting the ability to modify mBMI with lifestyle intervention. Our findings show that, the mBMI score captures information on metabolic dysregulation that is independent of the measured BMI and so provides an opportunity to assess metabolic health to identify "at risk" individuals for targeted intervention and monitoring.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

