Integrin signaling is critical for myeloid-mediated support of T-cell acute lymphoblastic leukemia
- PMID: 37805579
- PMCID: PMC10560206
- DOI: 10.1038/s41467-023-41925-z
Integrin signaling is critical for myeloid-mediated support of T-cell acute lymphoblastic leukemia
Abstract
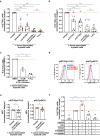
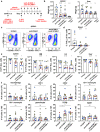
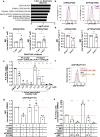
We previously found that T-cell acute lymphoblastic leukemia (T-ALL) requires support from tumor-associated myeloid cells, which activate Insulin Like Growth Factor 1 Receptor (IGF1R) signaling in leukemic blasts. However, IGF1 is not sufficient to sustain T-ALL in vitro, implicating additional myeloid-mediated signals in leukemia progression. Here, we find that T-ALL cells require close contact with myeloid cells to survive. Transcriptional profiling and in vitro assays demonstrate that integrin-mediated cell adhesion activates downstream focal adhesion kinase (FAK)/ proline-rich tyrosine kinase 2 (PYK2), which are required for myeloid-mediated T-ALL support, partly through activation of IGF1R. Blocking integrin ligands or inhibiting FAK/PYK2 signaling diminishes leukemia burden in multiple organs and confers a survival advantage in a mouse model of T-ALL. Inhibiting integrin-mediated adhesion or FAK/PYK2 also reduces survival of primary patient T-ALL cells co-cultured with myeloid cells. Furthermore, elevated integrin pathway gene signatures correlate with higher FAK signaling and myeloid gene signatures and are associated with an inferior prognosis in pediatric T-ALL patients. Together, these findings demonstrate that integrin activation and downstream FAK/PYK2 signaling are important mechanisms underlying myeloid-mediated support of T-ALL progression.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Vadillo E, Dorantes-Acosta E, Pelayo R, Schnoor M. T cell acute lymphoblastic leukemia (T-ALL): new insights into the cellular origins and infiltration mechanisms common and unique among hematologic malignancies. Blood Rev. 2018;32:36–51. - PubMed
-
- Hunger SP, Mullighan CG. Acute lymphoblastic leukemia in children. N. Engl. J. Med. 2015;373:1541–1552. - PubMed
-
- Möricke A, et al. Dexamethasone vs prednisone in induction treatment of pediatric ALL: results of the randomized trial AIEOP-BFM ALL 2000. Blood. 2016;127:2101–2112. - PubMed
-
- Luskin MR, et al. Nelarabine, cyclosphosphamide and etoposide for adults with relapsed T‐cell acute lymphoblastic leukaemia and lymphoma. Brit. J. Haematol. 2016;174:332–334. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

