Effects of sodium nitrite reduction, removal or replacement on cured and cooked meat for microbiological growth, food safety, colon ecosystem, and colorectal carcinogenesis in Fischer 344 rats
- PMID: 37805637
- PMCID: PMC10560221
- DOI: 10.1038/s41538-023-00228-9
Effects of sodium nitrite reduction, removal or replacement on cured and cooked meat for microbiological growth, food safety, colon ecosystem, and colorectal carcinogenesis in Fischer 344 rats
Abstract
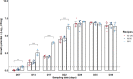
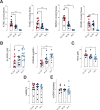
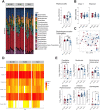
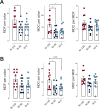
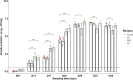
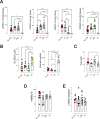
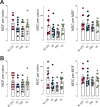
Epidemiological and experimental evidence indicated that processed meat consumption is associated with colorectal cancer risks. Several studies suggest the involvement of nitrite or nitrate additives via N-nitroso-compound formation (NOCs). Compared to the reference level (120 mg/kg of ham), sodium nitrite removal and reduction (90 mg/kg) similarly decreased preneoplastic lesions in F344 rats, but only reduction had an inhibitory effect on Listeria monocytogenes growth comparable to that obtained using the reference nitrite level and an effective lipid peroxidation control. Among the three nitrite salt alternatives tested, none of them led to a significant gain when compared to the reference level: vegetable stock, due to nitrate presence, was very similar to this reference nitrite level, yeast extract induced a strong luminal peroxidation and no decrease in preneoplastic lesions in rats despite the absence of NOCs, and polyphenol rich extract induced the clearest downward trend on preneoplastic lesions in rats but the concomitant presence of nitrosyl iron in feces. Except the vegetable stock, other alternatives were less efficient than sodium nitrite in reducing L. monocytogenes growth.
© 2023. Springer Nature Limited.
Conflict of interest statement
A.P., B.F., J.-L.M., S.J. and G.N. declare competing financial interests: they are employed by the French Pork Institute (IFIP). F.P., F.G. and V.S.-L. declare competing financial interests: some research projects from their academic research teams have been co-financed by the processed meat sector. C.B., N.N., E.F., V.B., J.D., P.P., C.H.T., L.T., E.K., N.P., L.A., V.T., M.O., G.C. and T.K. declare no competing financial or non-financial interests.
Figures

References
-
- Bouvard V, et al. Carcinogenicity of consumption of red and processed meat. Lancet Oncol. 2015;16:1599–1600. - PubMed
-
- IARC Working Group on the Evaluation of Carcinogenic Risk to Humans. Red Meat and Processed Meat. (International Agency for Research on Cancer, 2018). - PubMed
-
- WCRF/AICR. WCRF. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. (World Cancer Research Fund and American Institute for Cancer Research, 1997).
-
- WCRF/AICR. Continuous Update Project Expert Report 2018. Meat, fish and dairy products and the risk of cancer. (World Cancer Research Fund and American Institute for Cancer Research, 2018).
LinkOut - more resources
Full Text Sources

