On the regulation of human D-aspartate oxidase
- PMID: 37805834
- PMCID: PMC10588558
- DOI: 10.1002/pro.4802
On the regulation of human D-aspartate oxidase
Abstract
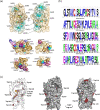
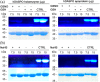
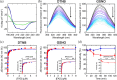
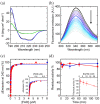
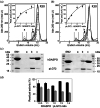
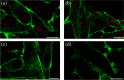
The human flavoenzyme D-aspartate oxidase (hDASPO) controls the level of D-aspartate in the brain, a molecule acting as an agonist of NMDA receptors and modulator of AMPA and mGlu5 receptors. hDASPO-induced D-aspartate degradation prevents age-dependent deterioration of brain functions and is related to psychiatric disorders such as schizophrenia and autism. Notwithstanding this crucial role, less is known about hDASPO regulation. Here, we report that hDASPO is nitrosylated in vitro, while no evidence of sulfhydration and phosphorylation is apparent: nitrosylation affects the activity of the human flavoenzyme to a limited extent. Furthermore, hDASPO interacts with the primate-specific protein pLG72 (a well-known negative chaperone of D-amino acid oxidase, the enzyme deputed to D-serine degradation in the human brain), yielding a ~114 kDa complex, with a micromolar dissociation constant, promoting the flavoenzyme inactivation. At the cellular level, pLG72 and hDASPO generate a cytosolic complex: the expression of pLG72 negatively affects the hDASPO level by reducing its half-life. We propose that pLG72 binding may represent a protective mechanism aimed at avoiding cytotoxicity due to H2 O2 produced by the hDASPO enzymatic degradation of D-aspartate, especially before the final targeting to peroxisomes.
Keywords: D-aspartate; flavooxidase; neurotransmission; pLG72; post-translational modification; protein-protein interaction.
© 2023 The Authors. Protein Science published by Wiley Periodicals LLC on behalf of The Protein Society.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Cappelletti P, Campomenosi P, Pollegioni L, Sacchi S. The degradation (by distinct pathways) of human D‐amino acid oxidase and its interacting partner pLG72‐two key proteins in D‐serine catabolism in the brain. FEBS J. 2014;281:708–723. - PubMed
-
- Cristino L, Luongo L, Squillace M, Paolone G, Mango D, Piccinin S, et al. D‐Aspartate oxidase influences glutamatergic system homeostasis in mammalian brain. Neurobiol Aging. 2015;36:1890–1902. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

