Development of a novel serological assay for the detection of mpox infection in vaccinated populations
- PMID: 37805977
- PMCID: PMC10686281
- DOI: 10.1002/jmv.29134
Development of a novel serological assay for the detection of mpox infection in vaccinated populations
Abstract
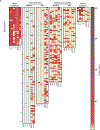
In 2022 the World Health Organization declared a Public Health Emergency for an outbreak of mpox, the zoonotic Orthopoxvirus (OPV) affecting at least 104 nonendemic locations worldwide. Serologic detection of mpox infection is problematic, however, due to considerable antigenic and serologic cross-reactivity among OPVs and smallpox-vaccinated individuals. In this report, we developed a high-throughput multiplex microsphere immunoassay using a combination of mpox-specific peptides and cross-reactive OPV proteins that results in the specific serologic detection of mpox infection with 93% sensitivity and 98% specificity. The New York State Non-Vaccinia Orthopoxvirus Microsphere Immunoassay is an important tool to detect subclinical mpox infection and understand the extent of mpox spread in the community through retrospective analysis.
Keywords: antibody; immunoassay; mpox; orthopoxvirus; serology.
© 2023 Wiley Periodicals LLC.
Conflict of interest statement
Declaration of Interests
P.K. and W.H. have a financial interest in Aalto Bio Reagents, a company that may have a commercial interest in the results of this research and technology. OHSU and M.K.S. have a financial interest in Najit Technologies, Inc., a company that may have a commercial interest in the results of this research and technology. This potential individual and institutional conflict of interest has been reviewed and managed by OHSU. The Icahn School of Medicine at Mount Sinai has filed patent applications relating to SARS-CoV-2 serological assays, which list Viviana Simon as a co-inventor.
Figures



Update of
-
Development of a Novel Serological Assay for the Detection of Mpox Infection in Vaccinated Populations.medRxiv [Preprint]. 2023 Apr 24:2023.04.18.23288419. doi: 10.1101/2023.04.18.23288419. medRxiv. 2023. Update in: J Med Virol. 2023 Oct;95(10):e29134. doi: 10.1002/jmv.29134. PMID: 37162953 Free PMC article. Updated. Preprint.
Similar articles
-
Development of a Novel Serological Assay for the Detection of Mpox Infection in Vaccinated Populations.medRxiv [Preprint]. 2023 Apr 24:2023.04.18.23288419. doi: 10.1101/2023.04.18.23288419. medRxiv. 2023. Update in: J Med Virol. 2023 Oct;95(10):e29134. doi: 10.1002/jmv.29134. PMID: 37162953 Free PMC article. Updated. Preprint.
-
Development and validation of a quantitative Orthopoxvirus immunoassay to evaluate and differentiate serological responses to Mpox infection and vaccination.EBioMedicine. 2025 Mar;113:105622. doi: 10.1016/j.ebiom.2025.105622. Epub 2025 Feb 22. EBioMedicine. 2025. PMID: 39987746 Free PMC article.
-
Mpox: emergence following smallpox eradication, ongoing outbreaks and strategies for prevention.Curr Opin Infect Dis. 2025 Jun 1;38(3):222-227. doi: 10.1097/QCO.0000000000001100. Epub 2025 Jan 29. Curr Opin Infect Dis. 2025. PMID: 39878084 Review.
-
Antibodies Induced by Smallpox Vaccination after at Least 45 Years Cross-React with and In Vitro Neutralize Mpox Virus: A Role for Polyclonal B Cell Activation?Viruses. 2024 Apr 17;16(4):620. doi: 10.3390/v16040620. Viruses. 2024. PMID: 38675961 Free PMC article.
-
Mpox diagnostics: Review of current and emerging technologies.J Med Virol. 2023 Jan;95(1):e28429. doi: 10.1002/jmv.28429. J Med Virol. 2023. PMID: 36571266 Free PMC article. Review.
Cited by
-
Serosurvey of Immunity to Monkeypox (Mpox) Virus Antigens in People Living with HIV in South Florida.Pathogens. 2023 Nov 15;12(11):1355. doi: 10.3390/pathogens12111355. Pathogens. 2023. PMID: 38003819 Free PMC article.
-
Clade Ib: a new emerging threat in the Mpox outbreak.Front Pharmacol. 2024 Dec 19;15:1504154. doi: 10.3389/fphar.2024.1504154. eCollection 2024. Front Pharmacol. 2024. PMID: 39749207 Free PMC article. Review.
-
Short-Lived Neutralizing Antibody Responses to Monkeypox Virus in Smallpox Vaccine-Naive Persons after JYNNEOS Vaccination.Emerg Infect Dis. 2025 Feb;31(2):237-245. doi: 10.3201/eid3102.241300. Epub 2024 Jan 10. Emerg Infect Dis. 2025. PMID: 39793541 Free PMC article.
-
Discordant performance of mpox serological assays.J Virol Methods. 2024 Sep;329:115004. doi: 10.1016/j.jviromet.2024.115004. Epub 2024 Aug 8. J Virol Methods. 2024. PMID: 39127186 Free PMC article.
-
Phylogeny-aware linear B-cell epitope predictor detects targets associated with immune response to orthopoxviruses.Brief Bioinform. 2024 Sep 23;25(6):bbae527. doi: 10.1093/bib/bbae527. Brief Bioinform. 2024. PMID: 39503522 Free PMC article.
References
-
- Rimoin AW, Mulembakani PM, Johnston SC, Lloyd Smith JO, Kisalu NK, Kinkela TL, Blumberg S, Thomassen HA, Pike BL, Fair JN, et al. (2010). Major increase in human monkeypox incidence 30 years after smallpox vaccination campaigns cease in the Democratic Republic of Congo. Proc Natl Acad Sci U S A 107, 16262–16267. 2010/09/02. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

