Preconception and developmental DEHP exposure alter liver metabolism in a sex-dependent manner in adult mouse offspring
- PMID: 37806616
- PMCID: PMC10842112
- DOI: 10.1016/j.tox.2023.153640
Preconception and developmental DEHP exposure alter liver metabolism in a sex-dependent manner in adult mouse offspring
Abstract
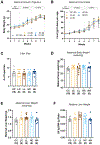
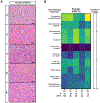
Environmental exposure to endocrine disrupting chemicals (EDCs) during critical periods of development is associated with an increased risk of metabolic diseases, including hepatic steatosis and obesity. Di-2-ethylhexyl-phthalate (DEHP) is an EDC strongly associated with these metabolic abnormalities. DEHP developmental windows of susceptibility are unknown yet have important public health implications. The purpose of this study was to identify these windows of susceptibility and determine whether developmental DEHP exposure alters hepatic metabolism later in life. Dams were exposed to control or feed containing human exposure relevant doses of DEHP (50 μg/kg BW/d) and high dose DEHP (10 mg/kg BW/d) from preconception until weaning or only exposed to DEHP during preconception. Post-weaning, all offspring were fed a control diet throughout adulthood. Using the Metabolon Untargeted Metabolomics platform, we identified 148 significant metabolites in female adult livers that were altered by preconception-gestation-lactation DEHP exposure. We found a significant increase in the levels of acylcarnitines, diacylglycerols, sphingolipids, glutathione, purines, and pyrimidines in DEHP-exposed female livers compared to controls. These changes in fatty acid oxidation and oxidative stress-related metabolites were correlated with hepatic changes including microvesicular steatosis, hepatocyte swelling, inflammation. In contrast to females, we observed fewer metabolic alterations in male offspring, which were uniquely found in preconception-only low dose DEHP exposure group. Although we found that preconception-gestational-lactation exposure causes the most liver pathology, we surprisingly found preconception exposure linked to an abnormal liver metabolome. We also found that two doses exhibited non-monotonic DEHP-induced changes in the liver. Collectively, these findings suggest that metabolic changes in the adult liver of offspring exposed periconceptionally to DHEP depends on the timing of exposure, dose, and sex.
Keywords: Developmental exposure; Di-2-ethylhexyl phthalate; Endocrine disrupting chemicals; Hepatic steatosis; Liver; Metabolomics.
Copyright © 2023 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




Similar articles
-
Developmental exposure to di(2-ethylhexyl) phthalate impairs endocrine pancreas and leads to long-term adverse effects on glucose homeostasis in the rat.Am J Physiol Endocrinol Metab. 2011 Sep;301(3):E527-38. doi: 10.1152/ajpendo.00233.2011. Epub 2011 Jun 14. Am J Physiol Endocrinol Metab. 2011. PMID: 21673306
-
NTP technical report on the toxicity studies of Dibutyl Phthalate (CAS No. 84-74-2) Administered in Feed to F344/N Rats and B6C3F1 Mice.Toxic Rep Ser. 1995 Apr;30:1-G5. Toxic Rep Ser. 1995. PMID: 12209194
-
A dose-response study following in utero and lactational exposure to di-(2-ethylhexyl)-phthalate (DEHP): non-monotonic dose-response and low dose effects on rat brain aromatase activity.Toxicology. 2006 Oct 29;227(3):185-92. doi: 10.1016/j.tox.2006.07.022. Epub 2006 Aug 1. Toxicology. 2006. PMID: 16949715
-
[Relationship of maternal malnutrition caused by Di(2-ethylhexyl) phthalate exposure with lifestyle disease in offspring].Nihon Eiseigaku Zasshi. 2012 Jan;67(1):22-5. doi: 10.1265/jjh.67.22. Nihon Eiseigaku Zasshi. 2012. PMID: 22449817 Review. Japanese.
-
Extracellular vesicles as a potential source of biomarkers for endocrine disruptors in MASLD: A short review on the case of DEHP.Biochimie. 2025 Jan;228:127-137. doi: 10.1016/j.biochi.2024.09.009. Epub 2024 Sep 21. Biochimie. 2025. PMID: 39307409 Review.
Cited by
-
Developmental programming: Sex-specific effects of prenatal exposure to a real-life mixture of environmental chemicals on liver function and transcriptome in sheep.Environ Pollut. 2025 Feb 15;367:125630. doi: 10.1016/j.envpol.2025.125630. Epub 2025 Jan 3. Environ Pollut. 2025. PMID: 39756566
References
-
- Endocrine-Disrupting Chemicals (EDCs). Published January 24, 2022. Accessed April 30, 2023. https://www.endocrine.org/patient-engagement/endocrine-library/edcs
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

