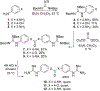
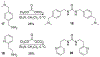Bisbenzamidine and Bisbenzguanidine Ureas Act as Antibacterial Agents against Pseudomonas aeruginosa
- PMID: 37806962
- PMCID: PMC10841437
- DOI: 10.1002/cmdc.202300496
Bisbenzamidine and Bisbenzguanidine Ureas Act as Antibacterial Agents against Pseudomonas aeruginosa
Abstract
Due to the global rise in the number of antibiotic resistant bacterial infections over the past 20 years, there is a dire need for the development of small molecule antibiotics capable of overcoming resistance mechanisms in pathogenic bacteria. Antibiotic development against Gram-negative pathogens, such as Pseudomonas aeruginosa, is especially challenging due to their additional outer membrane which reduces antibiotic entry. Recently, it has been shown that a broad range of nitrogen functionality, including guanidines, amidines, primary amines, imidazolines, and imidazoles, promote antibiotic and adjuvant activity in Gram-negative bacteria, but few of these have been targeted towards Pseudomonas aeruginosa specifically despite this pathogen being deemed a critical threat by the United States Centers for Disease Control and Prevention. Herein, we examined a small series of known and unknown nitrogenous dimers, with guanidine, amidine, dimethyl amine, and pyridine functionality, for antibacterial activity against multidrug resistant Pseudomonas aeruginosa. We found that two, with bisbenzguanidine and bisbenzamidine functionality, are potent against clinical isolates of multidrug resistant and biofilm forming Pseudomonas aeruginosa.
Keywords: amines; antibiotics; dimerization; pseudomonas aeruginosa.
© 2023 The Authors. ChemMedChem published by Wiley-VCH GmbH.
Figures
References
-
- Centers for Disease Control and Prevention (U.S.). Antibiotic Resistance Threats in the United States, 2019; Centers for Disease Control and Prevention (U.S.)
-
- Reynolds D, Kollef M, Drugs 2021. 81, 2117–2131; - PMC - PubMed
- Vincent JL, Sakr Y, Singer M, Martin-Loeches I, Machado FR, Marshall JC, Finfer S, Pelosi P, Brazzi L, Aditianingsih D, Timsit JF, Du B, Wittebole X, Máca J, Kannan S, Gorordo-Delsol LA, De Waele JJ, Mehta Y, Bonten MJM, Khanna AK, Kollef M, Human M, Angus DC, EPIC III Investigators, JAMA 2020. 323, 1478–1487. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous