Mitochondrial Fragmentation Promotes Inflammation Resolution Responses in Macrophages via Histone Lactylation
- PMID: 37807652
- PMCID: PMC10569354
- DOI: 10.1080/10985549.2023.2253131
Mitochondrial Fragmentation Promotes Inflammation Resolution Responses in Macrophages via Histone Lactylation
Abstract
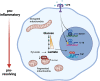
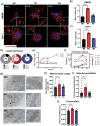
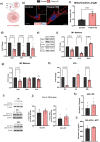
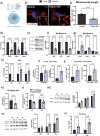
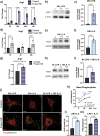
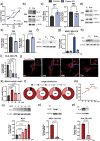
During the inflammatory response, macrophage phenotypes can be broadly classified as pro-inflammatory/classically activated "M1", or pro-resolving/alternatively "M2" macrophages. Although the classification of macrophages is general and assumes there are distinct phenotypes, in reality macrophages exist across a spectrum and must transform from a pro-inflammatory state to a proresolving state following an inflammatory insult. To adapt to changing metabolic needs of the cell, mitochondria undergo fusion and fission, which have important implications for cell fate and function. We hypothesized that mitochondrial fission and fusion directly contribute to macrophage function during the pro-inflammatory and proresolving phases. In the present study, we find that mitochondrial length directly contributes to macrophage phenotype, primarily during the transition from a pro-inflammatory to a proresolving state. Phenocopying the elongated mitochondrial network (by disabling the fission machinery using siRNA) leads to a baseline reduction in the inflammatory marker IL-1β, but a normal inflammatory response to LPS, similar to control macrophages. In contrast, in macrophages with a phenocopied fragmented phenotype (by disabling the fusion machinery using siRNA) there is a heightened inflammatory response to LPS and increased signaling through the ATF4/c-Jun transcriptional axis compared to control macrophages. Importantly, macrophages with a fragmented mitochondrial phenotype show increased expression of proresolving mediator arginase 1 and increased phagocytic capacity. Promoting mitochondrial fragmentation caused an increase in cellular lactate, and an increase in histone lactylation which caused an increase in arginase 1 expression. These studies demonstrate that a fragmented mitochondrial phenotype is critical for the proresolving response in macrophages and specifically drive epigenetic changes via lactylation of histones following an inflammatory insult.
Keywords: fission; fusion; histone lactylation; inflammation resolution; macrophages; mitochondrial metabolism.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
