Analysis of sodium phenylbutyrate and taurursodiol survival effect in ALS using external controls
- PMID: 37807839
- PMCID: PMC10723227
- DOI: 10.1002/acn3.51915
Analysis of sodium phenylbutyrate and taurursodiol survival effect in ALS using external controls
Abstract
Objective: Sodium phenylbutyrate and taurursodiol (PB and TURSO) was evaluated in amyotrophic lateral sclerosis (ALS) in the CENTAUR trial encompassing randomized placebo-controlled and open-label extension phases. On intent-to-treat (ITT) survival analysis, median overall survival (OS) was 4.8 months longer and risk of death 36% lower in those originally randomized to an initial 6-month double-blind period of PB and TURSO versus placebo. To estimate PB and TURSO treatment effect without placebo-to-active crossover, we performed a post hoc survival analysis comparing PB and TURSO-randomized participants from CENTAUR and a propensity score-matched, PB and TURSO-naïve external control cohort from the Pooled Resource Open-Access ALS Clinical Trials (PRO-ACT) database.
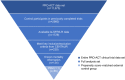
Methods: Clinical trial control participants from the PRO-ACT database who met prespecified eligibility criteria were propensity score matched 1:1 with PB and TURSO-randomized CENTAUR participants using prognostically significant covariates in ALS.
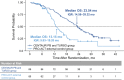
Results: Baseline characteristics including propensity score-matched covariates were generally well balanced between CENTAUR PB and TURSO (n = 89) and PRO-ACT external control (n = 85) groups. Estimated median (IQR) OS was 23.54 (14.56-39.32) months in the CENTAUR PB and TURSO group and 13.15 (9.83-19.20) months in the PRO-ACT external control group; hazard of death was 52% lower in the former group (hazard ratio, 0.48; 95% CI, 0.31-0.72; p = 0.00048).
Interpretation: This analysis suggests potentially greater survival benefit with PB and TURSO in ALS without placebo-to-active crossover than seen on ITT analysis in CENTAUR. Analyses using well-matched external controls may provide additional context for evaluating survival effects in future ALS trials.
© 2023 The Authors. Annals of Clinical and Translational Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
S. Paganoni reports research grants from the National Institutes of Health, Alector Therapeutics, Biohaven, Cytokinetics, Anelixis Pharmaceuticals, Revalesio Corporation, UCB, Clene, Prilenia, Seelos Therapeutics, Calico, and Denali Therapeutics unrelated to this manuscript; consulting fees from Amylyx Pharmaceuticals, Frequency Therapeutics, SOLA Pharmaceuticals, Stealth BioTherapeutics, Orion, Roche, Janssen, and Arrowhead; honoraria from Medscape; and board membership in the Association of Academic Physiatrists. M. Quintana and M. Vestrucci are employees of Berry Consultants, LLC, report consulting fees from Amylyx Pharmaceuticals for some of the analyses described in the submitted work, and serve as consultants to numerous additional pharmaceutical and device companies. A.V. Sherman reports grants from the National Institutes of Health, US Food and Drug Administration, the ALS Association, and ALS Finding a Cure® and has research contracts with Biogen, Amylyx, and Mitsubishi Tanabe Pharma America (MTPA). Y. Wu and J. Timmons are full‐time employees of and have stock options in Amylyx Pharmaceuticals, Inc. M. Cudkowicz reports consulting fees from Immunity Pharm Ltd, Cytokinetics, Takeda, Biogen, ALSpharma, RRD International, Transposon, QurAlis, Regeneron Pharmaceuticals, AB Science, Locust Walk, Servier, Vector Y, Roche, Novartis, Arrowhead Pharmaceuticals, VectorY Therapeutics, Servier, Pasithea Therapeutics, and Denali Therapeutics and serves on the board of directors for Praxis.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
