The gut microbiota metabolite butyrate mitigates MPTP/MPP+ -induced Parkinson's disease by inhibiting the JAK2/STAT3 signaling pathway
- PMID: 37807941
- PMCID: PMC11895885
- DOI: 10.1002/kjm2.12745
The gut microbiota metabolite butyrate mitigates MPTP/MPP+ -induced Parkinson's disease by inhibiting the JAK2/STAT3 signaling pathway
Abstract
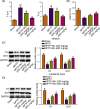
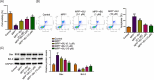
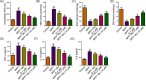
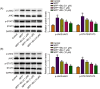
Butyrate (BU), a gut microbiota-derived metabolite, has been reported to play a neuroprotective role in Parkinson's disease (PD). However, the specific molecular mechanism of BU has not been fully interpreted. This work aimed to verify the protective effects of BU against MPTP/MPP+ -induced neurotoxicity and explore the mechanisms involved. The results showed that BU protected against MPTP-induced motor dysfunction and decreased tyrosine hydroxylase (TH) and dopamine transporter (DAT) levels. Additionally, BU pretreatment improved PC12 cell viability and reduced MPP+ -induced PC12 cell apoptosis. BU treatment also attenuated MPP+ -stimulated oxidative stress and inflammatory response in PC12 cells. Furthermore, BU inhibited MPTP/MPP+ -induced hyperactivation of the JAK2/STAT3 signaling in mice and PC12 cells. Besides, a JAK2 agonist, Coumermycin A1 (C-A1), substantially reversed BU-mediated inhibition on JAK2/STAT3 phosphorylation in MPP+ -challenged PC12 cells and abated BU-induced repression on MPP+ -triggered apoptosis, oxidative stress, and inflammatory response in PC12 cells. To sum up, BU might exert neuroprotective effects against MPP+ /MPTP-induced neurotoxicity by inactivating the JAK2/STAT3 signaling.
Keywords: JAK2/STAT3; Parkinson's disease; butyrate; gut microbiota metabolite.
© 2023 The Authors. The Kaohsiung Journal of Medical Sciences published by John Wiley & Sons Australia, Ltd on behalf of Kaohsiung Medical University.
Conflict of interest statement
All authors declare no conflict of interest.
Figures
Similar articles
-
Protection by rhynchophylline against MPTP/MPP+-induced neurotoxicity via regulating PI3K/Akt pathway.J Ethnopharmacol. 2021 Mar 25;268:113568. doi: 10.1016/j.jep.2020.113568. Epub 2020 Nov 11. J Ethnopharmacol. 2021. PMID: 33188898
-
Probiotics mixture increases butyrate, and subsequently rescues the nigral dopaminergic neurons from MPTP and rotenone-induced neurotoxicity.J Nutr Biochem. 2019 Jul;69:73-86. doi: 10.1016/j.jnutbio.2019.03.021. Epub 2019 Apr 6. J Nutr Biochem. 2019. PMID: 31063918
-
Paeonolum protects against MPP(+)-induced neurotoxicity in zebrafish and PC12 cells.BMC Complement Altern Med. 2015 Apr 29;15:137. doi: 10.1186/s12906-015-0661-0. BMC Complement Altern Med. 2015. PMID: 25925762 Free PMC article.
-
Ginsenoside Rk1 prevents 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson's disease via activating silence information regulator 3-mediated Nrf2/HO-1 signaling pathway.Hum Exp Toxicol. 2023 Jan-Dec;42:9603271231220610. doi: 10.1177/09603271231220610. Hum Exp Toxicol. 2023. PMID: 38105596
-
Narirutin reduces microglia-mediated neuroinflammation by inhibiting the JAK2/STAT3 pathway in MPP+/MPTP-induced Parkinson's disease models.Exp Neurol. 2025 Jul;389:115232. doi: 10.1016/j.expneurol.2025.115232. Epub 2025 Mar 30. Exp Neurol. 2025. PMID: 40169108
Cited by
-
The interplay between gut microbiota and the brain-gut axis in Parkinson's disease treatment.Front Neurol. 2024 May 29;15:1415463. doi: 10.3389/fneur.2024.1415463. eCollection 2024. Front Neurol. 2024. PMID: 38867886 Free PMC article. Review.
-
Interplay of human gastrointestinal microbiota metabolites: Short-chain fatty acids and their correlation with Parkinson's disease.Medicine (Baltimore). 2024 Apr 26;103(17):e37960. doi: 10.1097/MD.0000000000037960. Medicine (Baltimore). 2024. PMID: 38669388 Free PMC article. Review.
-
Gut microbiota and Parkinson's disease: potential links and the role of fecal microbiota transplantation.Front Aging Neurosci. 2024 Nov 29;16:1479343. doi: 10.3389/fnagi.2024.1479343. eCollection 2024. Front Aging Neurosci. 2024. PMID: 39679259 Free PMC article. Review.
-
Implications of Butyrate Signaling Pathways on the Motor Symptomatology of Parkinson's Disease and Neuroprotective Effects-Therapeutic Approaches: A Systematic Review.Int J Mol Sci. 2024 Aug 19;25(16):8998. doi: 10.3390/ijms25168998. Int J Mol Sci. 2024. PMID: 39201684 Free PMC article.
-
Understanding the Pre-Clinical Stages of Parkinson's Disease: Where Are We in Clinical and Research Settings?Int J Mol Sci. 2025 Jul 17;26(14):6881. doi: 10.3390/ijms26146881. Int J Mol Sci. 2025. PMID: 40725128 Free PMC article. Review.
References
-
- Wickremaratchi MM, Ben‐Shlomo Y, Morris HR. The effect of onset age on the clinical features of Parkinson's disease. Eur J Neurol. 2009;16:450–456. - PubMed
-
- Samii A, Nutt JG, Ransom BR. Parkinson's disease. Lancet. 2004;363:1783–1793. - PubMed
-
- Abu‐Elfotuh K, Hamdan AME, Mohammed AA, Atwa AM, Kozman MR, Ibrahim AM, et al. Neuroprotective effects of some nutraceuticals against manganese‐induced Parkinson's disease in rats: possible modulatory effects on TLR4/NLRP3/NF‐kappaB, GSK‐3beta, Nrf2/HO‐1, and apoptotic pathways. Pharmaceuticals (Basel). 2022;15:15. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

