Acute protein kinase C beta inhibition preserves coronary endothelial function after cardioplegic hypoxia/reoxygenation
- PMID: 37808045
- PMCID: PMC10556935
- DOI: 10.1016/j.xjon.2023.06.014
Acute protein kinase C beta inhibition preserves coronary endothelial function after cardioplegic hypoxia/reoxygenation
Abstract
Objective: Protein kinase C (PKC) influences myocardial contractility and susceptibility to long-term cardiac dysfunction after ischemia-reperfusion injury. In diabetes, PKC inhibition has a protective effect in terms of microvascular dysfunction. SK-channel dysfunction also influences endothelial dysfunction in cardioplegic hypoxia-reoxygenation (CP-H/R). Here, we examine whether acute inhibition of PKC beta protects against CP-H/R-induced coronary endothelial and SK channel dysfunction.
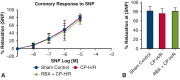
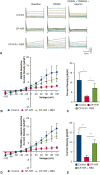
Methods: Isolated mouse coronary arterioles, half pretreated with selective PKC inhibitor ruboxistaurin (RBX), were subjected to hyperkalemic, cardioplegic hypoxia (1 hour), and reoxygenation (1 hour) with Krebs buffer. Sham control vessels were continuously perfused with oxygenated Krebs buffer without CP-H/R. After 1 hour of reoxygenation, responses to the endothelium-dependent vasodilator adenosine-diphosphate (ADP) and the SK-channel activator NS309 were examined. Endothelial SK-specific potassium currents from mouse heart endothelial cells were examined using whole-cell path clamp configurations in response to NS309 and SK channel blockers apamin and TRAM34.
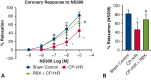
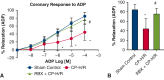
Results: CP-H/R significantly decreased coronary relaxation responses to ADP (P = .006) and NS309 (P = .0001) compared with the sham control group. Treatment with selective PKC beta inhibitor RBX significantly increased recovery of coronary relaxation responses to ADP (P = .031) and NS309 (P = .004) after CP-H/R. Treatment with RBX significantly increased NS309-mediated potassium currents following CP-H/R (P = .0415). Apamin and TRAM34 sensitive currents were significantly greater in CP-H/R + RBX versus CP-H/R mouse heart endothelial cells (P = .0027).
Conclusions: Acute inhibition of PKC beta significantly protected mouse coronary endothelial function after CP-H/R injury. This suggests that acute PKC beta inhibition may be a novel approach for preventing microvascular dysfunction during CP-H/R.
Keywords: cardioplegia; hypoxia–reoxygenation; protein kinase C; ruboxistaurin; vascular reactivity.
© 2023 The Author(s).
Figures
Similar articles
-
Coronary endothelial dysfunction prevented by small-conductance calcium-activated potassium channel activator in mice and patients with diabetes.J Thorac Cardiovasc Surg. 2020 Dec;160(6):e263-e280. doi: 10.1016/j.jtcvs.2020.01.078. Epub 2020 Feb 19. J Thorac Cardiovasc Surg. 2020. PMID: 32199659 Free PMC article.
-
Inhibition of mitochondrial reactive oxygen species improves coronary endothelial function after cardioplegic hypoxia/reoxygenation.J Thorac Cardiovasc Surg. 2022 Nov;164(5):e207-e226. doi: 10.1016/j.jtcvs.2021.06.029. Epub 2021 Jun 26. J Thorac Cardiovasc Surg. 2022. PMID: 34274141 Free PMC article.
-
Calcium-activated potassium channels contribute to human coronary microvascular dysfunction after cardioplegic arrest.Circulation. 2008 Sep 30;118(14 Suppl):S46-51. doi: 10.1161/CIRCULATIONAHA.107.755827. Circulation. 2008. PMID: 18824768 Free PMC article.
-
Cellular and molecular therapeutic targets for treatment of contractile dysfunction after cardioplegic arrest.Ann Thorac Surg. 1999 Nov;68(5):1934-41. doi: 10.1016/s0003-4975(99)01034-6. Ann Thorac Surg. 1999. PMID: 10585107 Review.
-
Poly(ADP-ribose) Polymerase (PARP) and PARP Inhibitors: Mechanisms of Action and Role in Cardiovascular Disorders.Cardiovasc Toxicol. 2018 Dec;18(6):493-506. doi: 10.1007/s12012-018-9462-2. Cardiovasc Toxicol. 2018. PMID: 29968072 Review.
Cited by
-
Protein Kinase C-β Inhibition and Survival Signaling after Simulated Cardioplegic-Ischemia/Reperfusion in Nondiabetic and Diabetic Human Coronary Arterial Endothelial Cells.J Am Coll Surg. 2025 Aug 1;241(2):118-135. doi: 10.1097/XCS.0000000000001248. Epub 2025 Jul 16. J Am Coll Surg. 2025. PMID: 39651752 Free PMC article.
-
Single Nuclei Transcriptomics Reveals Obesity-Induced Endothelial and Neurovascular Dysfunction: Implications for Cognitive Decline.Int J Mol Sci. 2024 Oct 17;25(20):11169. doi: 10.3390/ijms252011169. Int J Mol Sci. 2024. PMID: 39456952 Free PMC article.
References
-
- Carvajal C., Goyal A., Tadi P. StatPearls. StatPearls publishing; 2022. Cardioplegia. Accessed December 12, 2022. https://www.ncbi.nlm.nih.gov/books/NBK554463/ - PubMed
-
- Feng J., Kant S., Sellke F.W. Microvascular dysfunction following cardioplegic arrest and cardiopulmonary bypass. Vessel Plus. 2021;5:30. doi: 10.20517/2574-1209.2021.57. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

