Desialylated Platelet Clearance in the Liver is a Novel Mechanism of Systemic Immunosuppression
- PMID: 37808178
- PMCID: PMC10551749
- DOI: 10.34133/research.0236
Desialylated Platelet Clearance in the Liver is a Novel Mechanism of Systemic Immunosuppression
Abstract
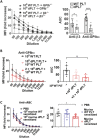
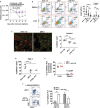
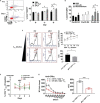
Platelets are small, versatile blood cells that are critical for hemostasis/thrombosis. Local platelet accumulation is a known contributor to proinflammation in various disease states. However, the anti-inflammatory/immunosuppressive potential of platelets has been poorly explored. Here, we uncovered, unexpectedly, desialylated platelets (dPLTs) down-regulated immune responses against both platelet-associated and -independent antigen challenges. Utilizing multispectral photoacoustic tomography, we tracked dPLT trafficking to gut vasculature and an exclusive Kupffer cell-mediated dPLT clearance in the liver, a process that we identified to be synergistically dependent on platelet glycoprotein Ibα and hepatic Ashwell-Morell receptor. Mechanistically, Kupffer cell clearance of dPLT potentiated a systemic immunosuppressive state with increased anti-inflammatory cytokines and circulating CD4+ regulatory T cells, abolishable by Kupffer cell depletion. Last, in a clinically relevant model of hemophilia A, presensitization with dPLT attenuated anti-factor VIII antibody production after factor VIII ( infusion. As platelet desialylation commonly occurs in daily-aged and activated platelets, these findings open new avenues toward understanding immune homeostasis and potentiate the therapeutic potential of dPLT and engineered dPLT transfusions in controlling autoimmune and alloimmune diseases.
Copyright © 2023 June Li et al.
Figures




References
-
- Xu XR, Zhang D, Oswald BE, Carrim N, Wang X, Hou Y, Zhang Q, Lavalle C, McKeown T, Marshall AH, et al. Platelets are versatile cells: New discoveries in hemostasis, thrombosis, immune responses, tumor metastasis and beyond. Crit Rev Clin Lab Sci. 2016;53(6):409–430. - PubMed
-
- Jackson SP. Arterial thrombosis--insidious, unpredictable and deadly. Nat Med. 2011;17(11):1423–1436. - PubMed
-
- Smyth SS, McEver RP, Weyrich AS. Platelet functions beyond hemostasis. J Thromb Haemost. 2009;7(11):1759–1766. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

