The antibacterial activity of a photoactivatable diarylacetylene against Gram-positive bacteria
- PMID: 37808276
- PMCID: PMC10556703
- DOI: 10.3389/fmicb.2023.1243818
The antibacterial activity of a photoactivatable diarylacetylene against Gram-positive bacteria
Abstract
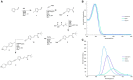
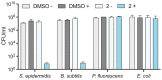
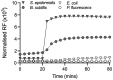
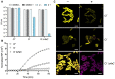
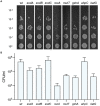
The emergence of antibiotic resistance is a growing threat to human health, and therefore, alternatives to existing compounds are urgently needed. In this context, a novel fluorescent photoactivatable diarylacetylene has been identified and characterised for its antibacterial activity, which preferentially eliminates Gram-positive over Gram-negative bacteria. Experiments confirmed that the Gram-negative lipopolysaccharide-rich outer surface is responsible for tolerance, as strains with reduced outer membrane integrity showed increased susceptibility. Additionally, bacteria deficient in oxidative damage repair pathways also displayed enhanced sensitivity, confirming that reactive oxygen species production is the mechanism of antibacterial activity. This new diarylacetylene shows promise as an antibacterial agent against Gram-positive bacteria that can be activated in situ, potentially for the treatment of skin infections.
Keywords: Gram-positive bacteria; antimicrobial resistance; lipopolysaccharides; photodynamic therapy; reactive oxygen species.
Copyright © 2023 Waite, Adams, Chisholm, Sims, Hughes, Dias, White, Welsby, Botchway, Whiting, Sharples and Ambler.
Conflict of interest statement
CTA, DC, CS, JH, ED, AW, and CAA were employed by the company LightOx Limited. CAA and AW own shares of LightOx Limited, the company licensed to pursue commercial applications of the novel chemicals described in this manuscript. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


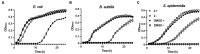

References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

