Antioxidant activity of glucosamine and its effects on ROS production, Nrf2, and O-GlcNAc expression in HMEC-1 cells
- PMID: 37808439
- PMCID: PMC10558709
- DOI: 10.1016/j.crtox.2023.100128
Antioxidant activity of glucosamine and its effects on ROS production, Nrf2, and O-GlcNAc expression in HMEC-1 cells
Abstract
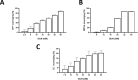
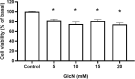
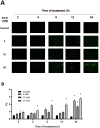
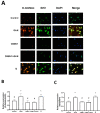
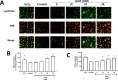
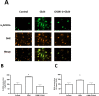
Glucosamine (GlcN) is the most used supplement for osteoarthritis treatment. In vitro studies have related GlcN to beneficial and detrimental effects on health. The aim of this study was to evaluate the effects of O-linked-N-acetylglucosaminylation (O-GlcNAc) on GlcN-induced ROS production and Nrf2 expression in human dermal microvascular endothelial cells-1 (HMEC-1) and to evaluate the antioxidant capacity of GlcN compared to well-known antioxidants. For this, we evaluate the antioxidant capacity by in vitro assays. Besides, the GlcN (5-20 mM) effects on cell viability, reactive oxygen species (ROS) production, O-GlcNAc, and nuclear factor erythroid-2-related factor 2 (Nrf2) expression with and without the O-GlcNAc inhibitor OSMI-1 (10 μM) in HMEC-1 were evaluated. GlcN showed high inhibitory concentration (low scavenging activity) against superoxide (O2•─, IC20 = 47.67 mM), 2,2-diphenyl-1-picrylhydrazyl (DPPH•, IC50 = 21.32 mM), and hydroxyl (HO•, IC50 = 14.04 mM) radicals without scavenging activity against hydrogen peroxide (H2O2) and low antioxidant capacity determined by oxygen radical absorbance capacity (ORAC, 0.001 mM Trolox equivalent) and ferric reducing antioxidant power (FRAP, 0.046 mM Trolox equivalent). In cell culture, GlcN (20 mM) reduced cell viability up to 26 % and induced an increase in ROS production (up to 70 %), O-GlcNAc (4-fold-higher vs. control), and Nrf2 expression (56 %), which were prevented by OSMI-1. These data suggest an association between O-GlcNAc, ROS production, and Nrf2 expression in HMEC-1 cells stimulated with GlcN.
Keywords: Endothelium; Glucosamine; Nrf2; O-GlcNAc; ROS production; Scavenging activity.
© 2023 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Glucosamine-induced Sp1 O-GlcNAcylation ameliorates hypoxia-induced SGLT dysfunction in primary cultured renal proximal tubule cells.J Cell Physiol. 2014 Oct;229(10):1557-68. doi: 10.1002/jcp.24599. J Cell Physiol. 2014. PMID: 24591095
-
Insulin and glucosamine infusions increase O-linked N-acetyl-glucosamine in skeletal muscle proteins in vivo.Metabolism. 1998 Apr;47(4):449-55. doi: 10.1016/s0026-0495(98)90058-0. Metabolism. 1998. PMID: 9550544
-
Protective effects of glucosamine and its acetylated derivative on serum/glucose deprivation-induced PC12 cells death: Role of reactive oxygen species.Res Pharm Sci. 2018 Apr;13(2):121-129. doi: 10.4103/1735-5362.223794. Res Pharm Sci. 2018. PMID: 29606966 Free PMC article.
-
Recent advancement of glucosamine and N-acetyl glucosamine production using microorganisms: A review.J Ind Microbiol Biotechnol. 2024 Dec 31;52:kuaf014. doi: 10.1093/jimb/kuaf014. J Ind Microbiol Biotechnol. 2024. PMID: 40424515 Free PMC article. Review.
-
Microbial production of glucosamine and N-acetylglucosamine: advances and perspectives.Appl Microbiol Biotechnol. 2013 Jul;97(14):6149-58. doi: 10.1007/s00253-013-4995-6. Epub 2013 Jun 11. Appl Microbiol Biotechnol. 2013. PMID: 23754704 Review.
Cited by
-
The Role of Oxidative Stress and Inflammation in the Pathogenesis and Treatment of Vascular Dementia.Cells. 2025 Apr 17;14(8):609. doi: 10.3390/cells14080609. Cells. 2025. PMID: 40277934 Free PMC article. Review.
-
10-Gingerol Increases Antioxidant Enzymes and Attenuates Lipopolysaccharide-Induced Inflammation by Modulating Adipokines in 3T3-L1 Adipocytes.Antioxidants (Basel). 2024 Sep 7;13(9):1093. doi: 10.3390/antiox13091093. Antioxidants (Basel). 2024. PMID: 39334752 Free PMC article.
References
-
- Cai X., Bao L., Ding Y., Dai X., Zhang Z., Li Y. Quercetin alleviates cell apoptosis and inflammation via the ER stress pathway in vascular endothelial cells cultured in high concentrations of glucosamine. Mol. Med. Rep. 2017;15(2):825–832. - PubMed
-
- Chen A.-S., Taguchi T., Sakai K., Kikuchi K., Wang M.-W., Miwa I. Antioxidant activities of chitobiose and chitotriose. Biol. Pharm. Bull. 2003;26(9):1326–1330. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

