In-stent restenosis after percutaneous coronary intervention: emerging knowledge on biological pathways
- PMID: 37808526
- PMCID: PMC10558044
- DOI: 10.1093/ehjopen/oead083
In-stent restenosis after percutaneous coronary intervention: emerging knowledge on biological pathways
Abstract
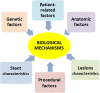
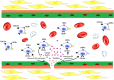
Percutaneous coronary intervention (PCI) has evolved significantly over the past four decades. Since its inception, in-stent restenosis (ISR)-the progressive reduction in vessel lumen diameter after PCI-has emerged as the main complication of the procedure. Although the incidence of ISR has reduced from 30% at 6 months with bare-metal stents to 7% at 4 years with drug-eluting stents (DESs), its occurrence is relevant in absolute terms because of the dimensions of the population treated with PCI. The aim of this review is to summarize the emerging understanding of the biological pathways that underlie ISR. In-stent restenosis is associated with several factors, including patient-related, genetic, anatomic, stent, lesion, and procedural characteristics. Regardless of associated factors, there are common pathophysiological pathways involving molecular phenomena triggered by the mechanical trauma caused by PCI. Such biological pathways are responses to the denudation of the intima during balloon angioplasty and involve inflammation, hypersensitivity reactions, and stem cell mobilization particularly of endothelial progenitor cells (EPCs). The results of these processes are either vessel wall healing or neointimal hyperplasia and/or neo-atherosclerosis. Unravelling the key molecular and signal pathways involved in ISR is crucial to identify appropriate therapeutic strategies aimed at abolishing the 'Achille's heel' of PCI. In this regard, we discuss novel approaches to prevent DES restenosis. Indeed, available evidence suggests that EPC-capturing stents promote rapid stent re-endothelization, which, in turn, has the potential to decrease the risk of stent thrombosis and allow the use of a shorter-duration dual antiplatelet therapy.
Keywords: Bare-metal stents; Drug-eluting stents; Endothelial progenitor cells; Hypersensitivity; In-stent restenosis; Inflammation; Percutaneous coronary intervention.
© The Author(s) 2023. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: All authors have nothing to disclose.
Figures





References
-
- Piccolo R, Giustino G, Mehran R, Windecker S. Stable coronary artery disease: revascularisation and invasive strategies. Lancet 2015;386:702–713. - PubMed
-
- Madhavan MV, Kirtane AJ, Redfors B, Généreux P, Ben-Yehuda O, Palmerini T, Benedetto U, Biondi-Zoccai G, Smits PC, von Birgelen C, Mehran R, McAndrew T, Serruys PW, Leon MB, Pocock SJ, Stone GW. Stent-related adverse events >1 year after percutaneous coronary intervention. J Am Coll Cardiol 2020;75:590–604. - PubMed
-
- Giustino G, Baber U, Sartori S, Mehran R, Mastoris I, Kini AS, Sharma SK, Pocock SJ, Dangas GD. Duration of dual antiplatelet therapy after drug-eluting stent implantation: a systematic review and meta-analysis of randomized controlled trials. J Am Coll Cardiol 2015;65:1298–1310. - PubMed
-
- Virmani R, Farb A. Pathology of in-stent restenosis. Curr Opin Lipidol 1999;10:499–506. - PubMed
-
- Otsuka F, Byrne RA, Yahagi K, Mori H, Ladich E, Fowler DR, Kutys R, Xhepa E, Kastrati A, Virmani R, Joner M. Neoatherosclerosis: overview of histopathologic findings and implications for intravascular imaging assessment. Eur Heart J 2015;36:2147–2159. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

